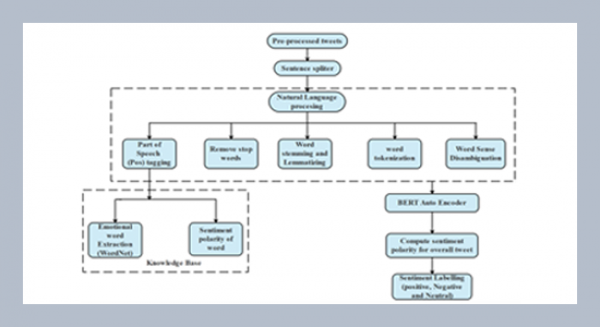
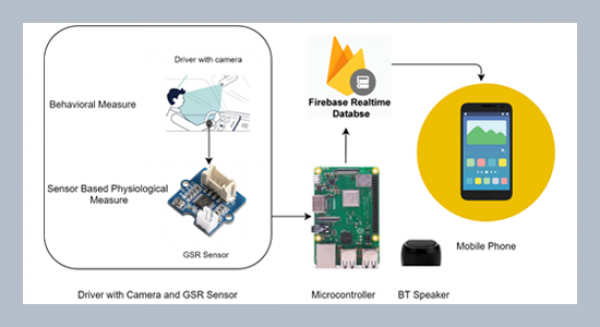
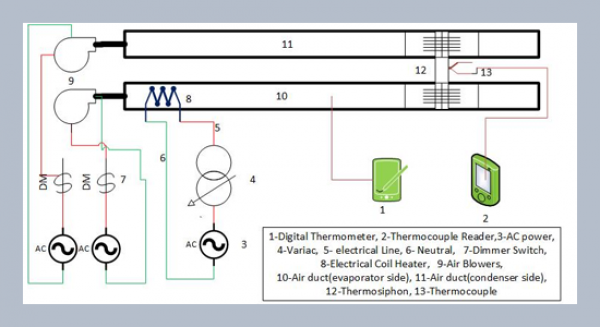
REFERENCES
- Abbas, M. 2020. Experimental investigation of activated carbon prepared from apricot stones material (ASM) adsorbent for removal of malachite green (MG) from aqueous solution, Adsorption Science & Technology, 38, 24–45.
- Akcil, A. 2010. A new global approach of cyanide management: international cyanide management code for the manufacture, transport, and use of cyanide in the production of gold, Mineral Processing & Extractive Metallurgy Review, 31, 135–149.
- Alatabe, M. jaafar, 2018. Crystallization in phase change materials, International Journal of Scientific Research in Science, Engineering and Technology, 4, 93–99.
- Alatabe, M. jaafar, 2018. A novel approach for adsorption of copper (II) ions from wastewater using cane papyrus, International Journal of Integrated Engineering, 10, 96–102.
- Alatabe, M.J. 2018. Adsorption of copper (II) ions from aqueous solution onto activated carbon prepared from cane papyrus, Pollution, 4, 649–662. doi: DOI: 10.22059/poll.2018.249931.377.
- Alatabe, M.J.A., Hussein, A. 2018. Adsorption of nickel ions from aqueaus solution using natural clay, Al-Nahrain Journal for Engineering Sciences, 21, 223–229.
- Alatabe, M.J.A., Kariem, N.O. 2019. Thorns, a novel natural plants for adsorption of lead (II) ions from wastewater equilibrium, isotherm, kinetics and thermodynamics, Eurasian Journal of Analytical Chemistry, 14, 163–174.
- Aliprandini, P., Veiga, M.M., Marshall, B.G., Scarazzato, T., Espinosa, D.C.R. 2020. Investigation of mercury cyanide adsorption from synthetic wastewater aqueous solution on granular activated carbon, Journal of Water Process Engineering, 34, 101154.
- Behnamfard, A., Salarirad, M.M. 2009. Equilibrium and kinetic studies on free cyanide adsorption from aqueous solution by activated carbon, Journal of hazardous materials, 170, 127–133.
- Brüger, A., Fafilek, G., Rojas-Mendoza, L. 2018. On the volatilisation and decomposition of cyanide contaminations from gold mining, Science of the Total Environment, 627, 1167–1173.
- Cheung, C.W., Porter, J.F., McKay, G. 2000. Elovich equation and modified second‐order equation for sorption of cadmium ions onto bone char, Journal of Chemical Technology & Biotechnology, 75, 963–970.
- Dai, X., Breuer, P.L., Jeffrey, M.I. 2010. Comparison of activated carbon and ion-exchange resins in recovering copper from cyanide leach solutions, Hydrometallurgy, 101, 48–57.
- Dai, X., Jeffrey, M.I., Breuer, P.L. 2010. A mechanistic model of the equilibrium adsorption of copper cyanide species onto activated carbon, Hydrometallurgy, 101, 99–107.
- Dai, X., Simons, A., Breuer, P. 2012. A review of copper cyanide recovery technologies for the cyanidation of copper containing gold ores, Minerals Engineering, 25, 1–13.
- Dash, R.R., Gaur, A., Balomajumder, C. 2009. Cyanide in industrial wastewaters and its removal: a review on biotreatment, Journal of hazardous materials, 163, 1–11.
- Donato, D.B., Madden-Hallett, D.M., Smith, G.B., Gursansky, W. 2017. Heap leach cyanide irrigation and risk to wildlife: Ramifications for the international cyanide management code, Ecotoxicology and environmental safety, 140, 271–278.
- Dunbar, K.R., Heintz, R.A. 1997. Chemistry of transition metal cyanide compounds: Modern perspectives, Progress in Inorganic Chemistry, 45, 283–392.
- Dwivedi, N., Balomajumder, C., Mondal, P. 2016. Comparative investigation on the removal of cyanide from aqueous solution using two different bioadsorbents, Water Resources and Industry, 15, 28–40.
- Eletta, O.A.A., Ajayi, O.A., Ogunleye, O.O., Akpan, I.C. 2016. Adsorption of cyanide from aqueous solution using calcinated eggshells: Equilibrium and optimisation studies, Journal of Environmental Chemical Engineering, 4, 1367–1375.
- Freundlich, H.M.F. 1906. Over the adsorption in solution, The Journal of Physical Chemistry, 57, 1100–1107.
- Gebresemati, M., Gabbiye, N., Sahu, O. 2017. Sorption of cyanide from aqueous medium by coffee husk: Response surface methodology, Journal of Applied Research and Technology, 15, 27–35.
- Guo, R., Chakrabarti, C.L., Subramanian, K.S., Ma, X., Lu, Y., Cheng, J., Pickering, W.F. 1993. Sorption of low levels of cyanide by granular activated carbon, Water Environment Research, 65, 640–644.
- Hadi, H.J., Al-zobai, K.M.M., Alatabe, M.J.A. 2020. Oil removal from produced water using imperata cylindrica as low-cost adsorbent, Current Applied Science and Technology, 494–511.
- Hattab, Z., Filali, N., Mazouz, R., Guerfi, K., Rebbani, N., Nafa, A., Kheriaf, S. 2016. Adsorption of cyanide ions in aqueous solution using raw and oxidized coke, Desalination and Water Treatment, 57, 3522–3531.
- Ho, Y.-S. 2006. Isotherms for the sorption of lead onto peat: comparison of linear and non-linear methods, Polish Journal of Environmental Studies, 15, 81–86.
- Ho, Y.-S., McKay, G. 1999. Pseudo-second order model for sorption processes, Process Biochem., 34, 451–465.
- Ho, Y.-S., Ofomaja, A.E. 2006. Biosorption thermodynamics of cadmium on coconut copra meal as biosorbent, Biochemical Engineering Journal, 30, 117–123.
- Hussein, A.A., Alatabe, M.J.A. 2019. Remediation of Lead-Contaminated soil, using clean energy in combination with Electro-Kinetic methods, Pollution, 5, 859–869.
- Ibragimova, R.I., Grebennikov, S.F., Gur’yanov, V.V., Kubyshkin, S.A., Vorob’ev-Desyatovskii, N.V. 2013. Adsorption of [Au(CN)2]− ions from aqueous solutions on an activated carbon surface, Protection of Metals and Physical Chemistry of Surfaces, 49, 402–407.
- Jaafar, M., Alatabe, A. 2019. Utilization of low cost adsorbents for the adsorption process of lead ions, International Journal of Modern Research in Engineering and Technology, 4, 29–48.
- Kaewkannetra, P., Imai, T., Garcia-Garcia, F.J., Chiu, T.Y. 2009. Cyanide removal from cassava mill wastewater using Azotobactor vinelandii TISTR 1094 with mixed microorganisms in activated sludge treatment system, Journal of hazardous materials, 172, 224–228.
- Kalipci, E., Namal, O.O. 2018. Removal of Cr(VI) using a novel adsorbent modification. Ultrasonic method with apricot kernel shells, Environment Protection Engineering, 44, 79–92.
- Krasilnikova, O.K., Artamonova, S.D., Voloshchuk, A.M., Yevsyukhin, A.E. 2005. Production of carbonaceous adsorbents from apricot kernels, Solid Fuel Chemistry, 39, 57–62.
- Kulig, K.W, Ballantyne, B. 1991. Cyanide toxicity. Atlanta, GA: U.S. Dept. of Health & Human Services, Public Health Service, Agency for Toxic Substances and Disease Registry, Print.
- Kurama, H., Çatalsarik, T. 2000. Removal of zinc cyanide from a leach solution by an anionic ion-exchange resin, Desalination, 129, 1–6.
- Langmuir, I. 1916. The constitution and fundamental properties of solids and liquids. Journal of the American Chemical Society, 38, 2221–2295.
- Langmuir, I. 1917. The constitution and fundamental properties of solids and liquids. II. Liquids., Journal of the American Chemical Society, 39, 1848–1906.
- Langmuir, I. 1918. The adsorption of gases on plane surfaces of glass, mica and platinum., Journal of the American Chemical Society, 40, 1361–1403.
- Liu, Y. 2009. Is the free energy change of adsorption correctly calculated?, Journal of Chemical & Engineering Data, 54, 1981–1985.
- Lu, D., Chang, Y., Wang, W., Xie, F., Asselin, E., Dreisinger, D. 2015. Copper and cyanide extraction with emulsion liquid membrane with LIX 7950 as the mobile carrier: part 1, Emulsion stability, Metals (Basel)., 5, 2034–2047.
- Mbadcam, J.K., Ngomo, H.M., Tcheka, C., Rahman, A.N., Djoyo, H.S., Kouotou, D. 2009. Batch equilibrium adsorption of cyanides from aqueous solution onto copper-and nickel-impregnated powder activated carbon and clay, Journal of Environmental Protection Scince, 3, 53–57.
- Meenakshi, S., Viswanathan, N. 2007. Identification of selective ion-exchange resin for fluoride sorption, Journal of Colloid and Interface Science, 308, 438–450.
- Namal, O.O., Kalipci, E. 2020. Adsorption kinetics of methylene blue removal from aqueous solutions using potassium hydroxide (KOH) modified apricot kernel shells, International Journal of Environmental Analytical Chemistry, 100, 1549–1565.
- Nourozi, R., NooriSepehr, M., Zarrabi, M. 2015. Adsorption of cyanide from aqueous solutions using magnetic hydroxyapatite nanoparticles synthesized by hydrothermal method: Equilibrium and kinetic study, Journal of Health, 5, 275–288.
- Pan, B., Xing, B. 2010. Adsorption kinetics of 17α-ethinyl estradiol and bisphenol A on carbon nanomaterials. I. Several concerns regarding pseudo-first order and pseudo-second order models, Journal of Soils and Sediments, 10, 838–844.
- Papari, F., Sahebi, S., Kouhgardi, E., Behresi, R., Hashemi, S., Asgari, G., Jorfi. S., Ramavandi, B. 2017. Cyanide adsorption from aqueous solution using mesoporous zeolite modified by cetyltrimethylammonium bromide surfactant., Desalination and Water Treatment, 97, 285–294.
- Parga, J.R., Shukla, S.S., Carrillo-Pedroza, F.R. 2003. Destruction of cyanide waste solutions using chlorine dioxide, ozone and titania sol, Waste Management, 23, 183–191.
- Paschka, M.G., Ghosh, R.S., Dzombak, D.A. 1999. Potential water-quality effects from iron cyanide anticaking agents in road salt, Water Environment Research, 71, 1235–1239.
- Saleh, T.A., Siddiqui, M.N., Al-Arfaj, A.A. 2016. Kinetic and intraparticle diffusion studies of carbon nanotubes-titania for desulfurization of fuels, Petroleum Science and Technology, 34, 1468–1474.
- Sarma, G.K., Gupta, S.S., Bhattacharyya, K.G. 2019. Nanomaterials as versatile adsorbents for heavy metal ions in water: a review, Environmental Science and Pollution Research, 26, 6245–6278.
- Sheremata, T.W., Hawari, J. 2000. Mineralization of RDX by the white rot fungus Phanerochaete chrysosporium to carbon dioxide and nitrous oxide, Environmental Science & Technology, 34, 3384–3388.
- Simonin, J.-P., Bouté, J. 2016. Intraparticle diffusion-adsorption model to describe liquid/solid adsorption kinetics, Revista mexicana de ingeniería química, 15, 161–173.
- Sun, B., Long, Y., Chen, Z., Liu, S., Zhang, H., Zhang, J., Han, W. 2014. Recent advances in flexible and stretchable electronic devices via electrospinning, Journal of Materials Chemistry C, 2, 1209–1219.
- Vadi, M., Abbasi, M., Zakeri, M., Jafari, Y.B. 2011. Application of the Freundlich Langmuir Temkin and Harkins-Jura adsorption isotherms for some amino acids and amino acids complexation with manganese ion (II) on carbon nanotube, International Conference on Nanotechnology and Biosensors IPCBEE vol.2 © (2011) IACSIT Press, Singapore.
- Vadi, M., Abbasi, M., Zakeri, M., Yazdi, B.J. 2010. Application of the Freundlich Langmuir Temkin and Harkins-Jura adsorption isotherms for some amino acids and amino acids complexation with manganese kn(ll) on carbon nanotube, Journal of Physical & Theoretical Chemistry, 7, 33–42.
- Wu, F.-C., Tseng, R.-L., Juang, R.-S. 2009. Characteristics of Elovich equation used for the analysis of adsorption kinetics in dye-chitosan systems, Chemical Engineering Journal, 150, 366–373.
- Xie, F., Wang, W. 2017. Recovery of copper and cyanide from waste cyanide solutions using emulsion liquid membrane with LIX 7950 as the carrier, Environmental Technology, 38, 1961–1968.
- Zhang, D., Ma, Y., Feng, H., Hao, Y. 2012. Adsorption of Cr (VI) from aqueous solution using carbon-microsilica composite adsorbent, Journal of the Chilean Chemical Society, 57, 964–968.
- Zhang, Q.-A., Wu, D.-D., Wei, C.-X. 2019. Purification of amygdalin from the concentrated Debitterizing-Water of apricot kernelsusing XDA-1 resin, Processes, 7, 359.