Shui-Wen Chang Chiena, Chun-Chia Huangb, and Min-Chao Wanga* Department of Environmental Engineering and Management, Chaoyang University of Technology, Wufeng, Taichung county 413, Taiwan, R.O.C.
Department of Soil and Environmental Sciences, National Chung Hsing University, Taichung 402, Taiwan, R.O.C.
Download Citation:
|
Download PDF
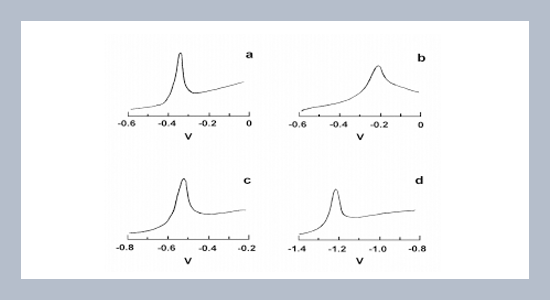
The humic substances including humic acid (HA) and fulvic acids (FAs) extracted from a refuse compost were investigated the average conditional concentration quotients of the complexes formed by the reaction of heavy metals with the humic substances. The characteristics of these humic substances were previously investigated by a related study. The concentrations of the free ions of Pb, Cu, Cd, and Zn in the reaction systems of heavy metal-HA suspensions and heavy metal-fulvic acid (FA) solutions were measured by anodic stripping voltammetry (ASV). The sequence of the average conditional concentration quotients of the complexes formed from the reaction of humic substances with heavy metals was FA (MW<1000) > FA (MW>1000) > HA (MW>1000). The sequence of reacting heavy metals with humic substances was Pb > Cu > Cd > Zn. Reactions of refuse compost-derived humic substances with heavy metals thus affect the mobility and biotoxicities of heavy metals in soil and the associated environments.ABSTRACT
Keywords:
humic substances; humic acid; fulvic acid; refuse compost; average conditional con-centration quotients; anodic stripping voltammetry.
Share this article with your colleagues
[1] Chang Chien, Shui-Wen, Huang, Chun-Chia, and Wang, Min-Chao. 2003. Analytical and spectroscopic characteristics of refuse compost-derived humic substances. International Journal of Journal of Applied Science and Engineering, 1, 1: 62-71.REFERENCES
[2] Sposito, G. 1986. Sorption of trace metals by humic materials in soils and natural waters. CRC Critical Reviews in Environmental Control, 16: 193-229.
[3] Stevenson, F. J. 1994. “Humus Chemistry: Genesis, Composition, Reactions”. 2nd Ed. John Wiley & Sons, New York.
[4] Schnitzer, M. 1969. Reaction between fulvic acid, a soil humic compound and inorganic soil constituents. Soil Science Society of America Proceeding, 33: 75-81.
[5] Gamble, D. S., Schnitzer, M., and Hoffman, 1970. Cu2+-Fulvic acid chelating equilibrium in 0.1 M KCl at 25 oC. Canadian Journal of Chemistry, 48: 3197-3204.
[6] Vinkler, P., Lakatos, B., and Meisel., J. 1976. Infrared spectroscopic investigations of humic substances and their metal complexes. Geoderma, 15: 231-242.
[7] Piccolo, A. and Stevenson, F. J. 1981. Infrared spectra of Cu2+, Pb2+, and Ca2+ complexes of soil humic substances. Geoderma, 27: 195-208.
[8] Chubin, R. G. and Street, J. J. 1981. Adsorption of cadmium on soil constituents in the presence of complexing ligands. Journal of Environmental Quality, 10: 225-228.
[9] Gregor, J. E. and Powell, H. K. J. 1988. Application of sampled - d. c. Anodic stripping votammetry to metal/fulvic acid equilibria. Analytica Chimica Acta, 211: 141-154.
[10] Perdue, E. M. 1985. Acidic functional groups of humic sbstances. “Humic Substances in Soil, Sediment, and Water”. John Wiley & Sons, New York: 493-526.
[11] Aiken, G. R., McKnight, D. M., We-shaw, R. L., and MacCarthy, P. 1985. An introduction to humic substances in soil, sediment, and water. “Humic substances in soil, sediment, and water”. John Wiley & Sons, New York: 1-9.
[12] Zen, J. M., Hsu, F. S., Chi, N. Y., Huang, S.Y., and Chung, M. J. 1995. Effect of model organic compounds on square-wave voltammetric stripping analysis at the nafion/chelating mercury film electrodes. Analytica Chimica Acta, 310: 407-417.
[13] Garrels, R. M. and Christ, C. L. 1965. Eh-pH diagrams. “Solutions, minerals, and equilibria”. Harper and Row, New York: 172-266.
[14] Lindsay, W. L. 1979. Zinc, Copper, Cadmium, Lead. “Chemical Equilibria in Soils”. Wiley-Interscience, New York: 211-220, 221-237, 315-327, 328-342.
[15] SAS Institute. 1996. “The SAS System for Windows”. Release 6.12. SAS Institute, Cary, NC.
[16] Steel, R. G. D. and Torrie, J. H. 1980. Duncan’s new Multiple-Range Test. “Principles and Procedures of Statistics”. McGraw-Hill, New York: 187-188.
[17] Schnitzer, M. and Khan, S. U. 1972. Characterization of humic substance by chemical methods. “Humic substances in the Environment”. Marcel Dekker, New York: 29-54.
[18] Sposito, G., Holtzclaw, K. M., LeVesque, C. S., and Johnston, C. T. 1982. Trace metal chemistry in arid-zone field soils amended with sewage sludge: II. Comparative study of the fulvic acid fraction. Soil Science Society of America Journal, 46: 265-270.
[19] Perdue, E. M. 1988. Measurements of binding site concentrations in humic substances. “Metal Speciation: Theory, Analysis and Application”. Lewis Publishers, Chelsea, Michigan: 135-154.
[20] Rashid, M. A. 1974. Adsorption of metals on sedimentary and peat humic acids. Chemical Geology, 13: 115-123.
[21] Beveridge, A. and Pickering, W. F. 1985. Influence of humate-solute interaction on aqueous heavy metal ion levels. Water, Air, and Soil Pollution, 14: 171-185.
ARTICLE INFORMATION
Accepted:
2003-07-22
Available Online:
2003-09-01
Chang Chien, S.-W., Huang, C.-C., Wang, M.-C. 2003. Reactions of refuse Compost-Derived humic substances with Lead, Copper, Cadmium, and Zinc, International Journal of Applied Science and Engineering, 1, 137–147. https://doi.org/10.6703/IJASE.2003.1(2).137
Cite this article: