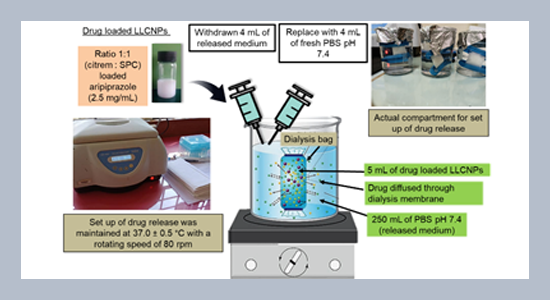
REFERENCES
- Akbarzadeh, A., Rezaei-Sadabady, R., Davaran, S., Joo, S.W., Zarghami, N., Hanifehpour, Y., Samiei, M., Kouhi., M., Nejati-Koshki, K. 2013. Liposome: classification, preparation, and applications. Nanoscale Research Letters, 8, 102.
- Ardiana, F., Lestari, M.L.A.D., Indrayanto, G. 2013. Aripiprazole: In profiles of drug substances, excipients and related methodology, 38, 2–501.
- Asha Spandana, A.K.M., Natarajan, J., Thirumaleshwar, S., Hemanth Kumar, S. 2020. A review of the preparation, characterization and application of nanostructured lipid carriers. International Journal of Research in Pharmaceutical Sciences, 11, 1130–1135.
- Ayala, A.P., Honorato, S.B., Filho, J.M., Grillo, D., Quintero, M., Gilles, F., Polla, G. 2010. Thermal stability of aripiprazole monohydrate investigated by raman spectroscopy. Vibrational Spectroscopy, 54, 169–173.
- Azmi, I.D.M., Wibroe, P.P., Wu, L.P., Kazem, A.I., Amenitsch, H., Moghimi, S.M., Yaghmur, A. 2016. A structurally diverse library of safe-by-design citrem-phospholipid lamellar and non-lamellar liquid crystalline nano-assemblies. Journal of Controlled Release, 239, 1–9.
- Babu, C., Kumara Babu, P., Sudhakar, K., Subha, M.C.S., Chowdoji Rao, K. 2014. Aripiprazole-loaded PLGA nanoparticles for controlled release studies: Effect of co-polymer ratio. International Journal of Drug Delivery, 6, 151–155.
- Badr-Eldin, S.M., Ahmed, T.A., Ismail, H.R. 2013. Aripiprazole-cyclodextrin binary systems for dissolution enhancement: Effect of preparation technique, cyclodextrin type and molar ratio. Iranian Journal of Basic Medical Sciences, 16, 1223–1231.
- Bastos, M. 2016. Using DSC to characterize thermotropic phase transitions in lipid bilayer membranes: The basics of liposome sample preparation and DSC studies. Malvern Instruments Limited, 1–21.
- Begam, M., Datta, V.M., Gowda, D.V., Aravindaram, A.S., Siddaramiah. 2014. Development and characterization of co-ground mixtures and solid dispersions of aripiprazole with hydrophilic carriers. International Journal of Pharmacy and Pharmaceutical Sciences, 6, 552–557.
- Bhosale, R., Osmani, R.A., Harkare, B.R., Ghodake, P.P. 2013. Cubosomes: The inimitable nanoparticulate drug carriers. Scholars Academic Journal of Pharmacy, 2, 481–486.
- Bonate, P.L. 2011. Pharmacokinetics. Wiley Interdisciplinary Reviews: Computational Statistics, 3, 332–342.
- Braun, D.E., Gelbrich, T., Kahlenberg, V., Tessadri, R., Wieser, J., Griesser, U.J. 2009. Conformational polymorphism in aripiprazole: preparation, stability and structure of five modifications. Journal of Pharmaceutical Sciences, 98, 2010–2026.
- Cagel, M., Tesan, F.C., Bernabeu, E., Salgueiro, M.J., Zubillaga, M.B., Moretton, M.A., Chiappetta, D.A. 2017. Polymeric mixed micelles as nanomedicines: Achievements and perspectives. European Journal of Pharmaceutics and Biopharmaceutics, 113, 211–228.
- Cai, L., Lin, C., Yang, N., Huang, Z., Miao, S., Chen, X., Pan, J., Rao, P., Liu, S. 2017. Preparation and characterization of nanoparticles made from co-incubation of SOD and glucose. Nanomaterials, 7, 1–11.
- Cerpnjak, K., Zvonar, A., Gasperlin, M., Vrecer, F. 2013. Lipid based system as a promising approach for enhancing the bioavailability of poor water-soluble drugs. Acta Pharmaceutica, 63, 427–445.
- Chandrashekhar V.K. 2012. Lipid crystallization: From self-assembly to hierarchical and biological ordering. Nanoscale, 4, 5779–5791.
- Chime, S.A. Onyishi, I.V. 2013. Lipid-based drug delivery systems (LDDS): Recent advances and applications of lipids in drug delivery. African Journal of Pharmacy and Pharmacology, 7, 3034–3059.
- Chong, J.Y.T., Mulet, X., Boyd, B.J., Drummond, C.J. 2015. Steric stabilizers for cubic phase lyotropic liquid crystal nanodispersions (cubosomes). In Advances in Planar Lipid Bilayers and Liposomes, 21, 131–187.
- Chountoulesi, M., Perinelli, D.R., Forys, A., Trzebicka, B., Pispas, S., Demetzos, C. 2020. Liquid crystalline nanoparticles for drug delivery: the role of gradient and block copolymers on the morphology, internal organization and release profile. European Journal of Pharmaceutics and Biopharmaceutics, 1–62.
- Chountoulesi, M., Pippa, N., Pispas, S., Chrysina, E.D., Forys, A., Trzebicka, B., Demetzos, C. 2018. Cubic lyotropic liquid crystals as drug delivery carriers: Physicochemical and morphological studies. International Journal of Pharmaceutics, 550, 57–70.
- Dutta, L., Mukherjee, B., Chakraborty, T., Das, M.K., Mondal, L., Bhattacharya, S., Gaonkar, R.H., Debnath, M. C. 2018. Lipid-based nanocarrier efficiently delivers highly water soluble drug across the blood–brain barrier into brain. Drug Delivery, 25, 504–516.
- Elezaby, R.S., Gad, H.A., Metwally, A.A., Geneidi, A.S., Awad, G.A. 2017. Self-assembled amphiphilic core-shell nanocarriers in line with the modern strategies for brain delivery. Journal of Controlled Release, 261, 43–61.
- Gabr, M.M., Mortada, S.M., Sallam, M.A. 2017. Hexagonal liquid crystalline nanodispersions proven superiority for enhanced oral delivery of rosuvastatin: In vitro characterization and in vivo pharmacokinetic study. Journal of Pharmaceutical Sciences, 106, 3103–3112.
- Guo, C., Wang, J., Cao, F., Lee, R.J., Zai, G. 2010. Lyotropic liquid crystal systems in drug delivery. Drug Delivery Today, 15, 1032–1040.
- Huang, Y., Gui, S. 2018. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Advances, 8, 6978–6987.
- Huang, Y., Gui, S. 2018. Factors affecting the structure of lyotropic liquid crystals and the correlation between structure and drug diffusion. RSC Advances, 8, 6978–6987.
- Kalepu, S., Nekkanti, V. 2015. Insoluble drug delivery strategies: review of recent advances and business prospects. Acta Pharmaceutica Sinica B, 5, 442–453.
- Khani, S., Keyhanfar, F., Amani, A. 2016. Design and evaluation of oral nanoemulsion drug delivery system of mebudipine, Drug Delivery, 23, 2035–2043.
- Krishna Sailaja, A., Amareshwar, P., Chakravarty, P. 2011. Formulation of solid lipid nanoparticles and their applications. Current Pharma Research, 1, 197–203.
- Kumar, A., Singh, H., Mishra, A., Mishra, A.K. 2020. Aripiprazole: An FDA Approved bioactive compound to treat schizophrenia- A mini review. Current Drug Discovery Technologies, 17, 23–29.
- Kumar, V.V., Chandrasekar, D., Ramakrishna, S., Kishan, V., Rao, Y.M., Diwan, P.V. 2007. Development and evaluation of nitrendipine loaded solid lipid nanoparticles: Influence of wax and glyceride lipids on plasma pharmacokinetics. International Journal of Pharmaceutics, 335, 167–175.
- Kumbhar, S.A., Kokare, C.R., Shrivastava, B., Gorain, B., Choudhury, H. 2021. Antipsychotic potential and safety profile of TPGS-based mucoadhesive aripiprazole nanoemulsion: Development and optimization for nose-to-brain delivery. Journal of Pharmaceutical Sciences, 110, 1761–1778.
- Larsson, M., Hill, A., Duffy, J. 2012. Suspension stability: Why particle size, zeta potential and rheology are important. Annual Transactions of the Nordic Rheology Society, 20, 209–214.
- Lertxundi, U., Hernandez, R., Medrano, J., Domingo-Echaburu, S., Garcia, M., Aguirre, C. 2018. Aripiprazole and impulse control disorders: Higher risk with the intramuscular depot formulation? International Clinical Psychopharmacology, 33, 56–58.
- Liu, K., Feng, Z., Shan, L., Yang, T., Qin, M., Tang, J., Zhang, W. 2017. Preparation, characterization, and antioxidative activity of bletilla striata polysaccharide/ chitosan microspheres for oligomeric proanthocyanidins. Drying Technology, 35, 1629–1643.
- Longo, A., Carotenuto, G., Palomba, M., De Nicola, S. 2011. Dependence of optical and microstructure properties of thiol-capped silver nanoparticles embedded in polymeric matrix. Polymers, 3, 1794–1804.
- Luangtana-Anan, M., Limmatvapirat, S., Nunthanid, J., Chalongsuk, R., Yamamoto, K. 2010. Polyethylene glycol on stability of chitosan micro-particulate carrier for protein. AAPS Pharm SciTech, 11, 1376–1382.
- Mahbubul, I.M., Saidur, R., Amalina, M.A., Elcioglu, E.B., Okutucu-Ozyurt, T. 2015. Effective ultrasonication process for better colloidal dispersion of nanofluid. Ultrasonic Sonochemistry, 26, 361–369.
- Malheiros, B., Dias de Castro, R., Lotierzo, M.C.G., Casadei, B.R., Barbosa, L.R.S. 2021. Design and manufacturing of monodisperse and malleable phytantriol-based cubosomes for drug delivery applications. Journal of Drug Delivery Science and Technology, 61, 102149.
- Maslizan, M., Azmi, I.D.M., Jaafar, A.M., Haris, M.S. 2021. Nanotechnology for molecular imaging of atherosclerosis: Current design and approaches. Journal of Advanced Research in Fluid Mechanics and Thermal Sciences, 81, 124–138.
- Modi, S., Anderson, B.D. 2013. Determination of drug release kinetics from nanoparticles: Overcoming pitfalls of the dynamic dialysis method. Molecular Pharmaceutics, 10, 3076–3089.
- Nethravani, G., Kishore Babu, M., Kalyani, V., Dakshinamurthy, D.V., Murali Krishna, G.V. 2014. Formulation and evaluation of aripiprazole-loaded liposomes for brain drug delivery. Journal of Chemical and Pharmaceutical Sciences, 7, 14–20.
- Olson, E. n.d. Zeta potential and colloidal chemistry. Particle Technology Labs, www.particletechlabs.com.
- Piazzini, V., Landucci, E., Urru, M., Chiarugi, A., Pellegrini-Giampietro, D.E., Bilia, A.R., Bergonzi, M.C. 2020. Enhanced dissolution, permeation and oral bioavailability of aripiprazole mixed micelles: In vitro and in vivo evaluation. International Journal of Pharmaceutics, 583, 119361.
- Popova, A.V., Hincha, D.K. 2011. Thermotropic phase behavior and headgroup interactions of the nonbilayer lipids phosphatidylethanolamine and monogalactosyldiacylglycerol in the dry state. BMC Biophysics, 4, 1–11.
- Saari, N.A.N., Mislan, A.A., Hashim, R., Zahid, N.I. 2018. Self-assembly, thermotropic, and lyotropic phase behavior of guerbet branched-chain maltosides. Langmuir, 34, 8962–8974.
- Savjani, K.T., Gajjar, A.K., Savjani, J.K. 2012. Drug solubility: Importance and enhancement techniques. International Scholarly Research Network Pharmaceutics, 1–10.
- Shirley, M., Perry, C.M. 2014. Aripiprazole (ABILIFY MAINTENA®): A review of its use as maintenance treatment for adult patients with schizophrenia. Drugs, 74, 1097–1110.
- Silki, Sinha, V.R. 2018. Enhancement of in vivo efficacy and oral bioavailability of aripiprazole with solid lipid nanoparticles. AAPS Pharm SciTech, 19, 1264–1273.
- Socrates, G. 2004. Infrared and raman characteristic group frequencies. Journal of Raman Spectroscopy, 35, 905.
- Spicer, P.T. 2004. Cubosomes: Bicontinuous liquid crystalline nanoparticles. Dekker Encyclopedia of Nanoscience and Nanotechnology, 881–892.
- Stuart, B.H. 2004. Infrared spectroscopy: Fundamentals and applications.
- Tan, A., Hong, L., Du, J.D., Boyd, B.J. 2018. Self-assembled nanostructured lipid systems: Is there a link between structure and cytotoxicity? Advanced Science, 6, 1–21.
- Thadanki, M., Kumari, P.S., Prabha, K.S. 2011. Overview of cubosomes: A nanoparticle. International Journal of Research in Pharmacy and Chemistry, 1, 535–541.
- Uner, M. 2006. Preparation, characterization and physico-chemical properties of Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC): Their benefits as colloidal drug carrier system. Pharmazie, 61, 375–386.
- William, H.D., Trevaskis, N.L., Charman, S.A., Shanker, R.M., Charman, W.N., Pouton, C.W., Christopher J. H. Porter. 2013. Strategies to address low drug solubility in discovery and development. Pharmacology Revision, 65, 315–499.
- Xu, Y., Liu, X., Lian, R., Wu, W. 2012. Enhanced dissolution and oral bioavailability of aripiprazole nanosuspension prepared by nanoprecipitation/ homogenization based on acid-base neutralization. International Journal of Pharmaceutics, 438, 287–295.
- Yaghmur, A., Mu, H. 2021. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharmaceutica Sinica B, 11, 871–885.
- Zhai, J., Tran, N., Sarkar, S., Fong, C., Mulet, X., Drummond, C. 2017. Self-assembled lyotropic liquid crystalline phase behavior of monoolein-capric acid-phospholipid nanoparticulate systems. Langmuir, 33, 2571–2580.