REFERENCES
- [1] Carsberg, C. J., Warenius H. M., and Friedmann, P. S. 1994. Ultraviolet radiation-induced melanogenesis in human melanocytes. Effects of modulating protein kinase C. Journal of Cell Science, 107: 2591-2597.
- [2] Gallarate, M., Carlotti, M. E., Trotta, M., and Bovo, S. 1999. On the stability of ascorbic acid in emulsified systems for topical and cosmetic use. International Journal of Pharmaceutics, 188: 233-241.
- [3] Murad, S., Grove, D., Lindberg, K. A., Reynolds, G., Sivarajah, A., and Pinnell, S. R. 1981. Regulation of collagen synthesis by ascorbic acid. Proceedings of the National Academy of Sciences of the United States of America, 78: 2879-2882.
- [4] Kuo, P. C., Damu, A. G., Cherng, C. Y., Jeng, J. F., Teng, C. M., Lee, E. J., and Wu, T. S. 2005. Isolation of a natural antioxidant, dehydrozingerone from Zingiber officinale and synthesis of its analogues for recognition of effective antioxidant and antityrosinase agents. Archives of Pharmacal Research, 28: 518–528.
- [5] Parrish, F. W., Wiley, B. J., Simmons, E. G., and Long, L. J. 1966. Production of aflatoxins and kojic acid by species of Aspergillus and Penicillium. Applied Microbiology, 14: 139.
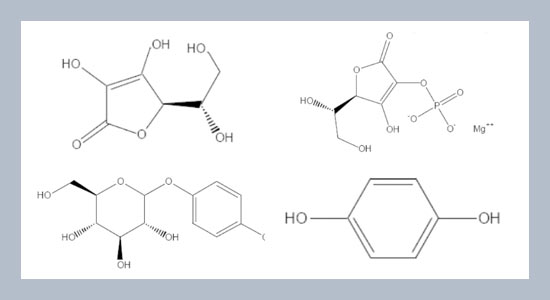
- [6] Burdock, G. A. Soni, M. G., and Carabin, I. G. 2001. Evaluation of health aspects of kojic acid in food. Regulatory Toxicology and Pharmacology, 33: 80-101.
- [7] Maeda, K. and Fukuda, M. 1991. In vitro effectiveness of several whitening cosmetic components in human melanocytes. Journal of Cosmetic Chemistry, 42: 361-368.
- [8] Kahn, V., Ben-Shalom, N., and Zakin, V. 1997. Effect of kojic acid on the oxidation of N-acetyldopamine by mushroom tyrosinase. Journal of Agricultural and Food Chemistry, 45: 4460-4465.
- [9] Noh, J. M., Kwak, S. Y., Seo, H. S., Seo, J. H., Kim, B. G., and Lee, Y. S. 2009. Kojic acid-amino acid conjugates as tyrosinase inhibitors. Bioorg. Medicinal Chemistry Letters, 19: 5586-5589.
- [10] Uher, M., Brtko, J., Rajniakova, O., Kovac, M., and Novotana, E. 1993. Kojic acid and its derivatives in cosmetics and health protection. Parfumerie und Kosmetik, 74: 554-556.
- [11] Parvez, S., Kang, M., Chung, H. S., and Bae, H. 2007. Naturally occurring tyrosinase inhibitors: mechanism and applications in skin health, cosmetics and agriculture industries. Phytotherapy research, 21: 805-816.
- [12] Lin, J. W., Chiang, H. M., Lin, Y. C., and Wen, K. C. 2008. Natural products with skin-whitening effects. Journal of Food and Drug Analysis, 16: 1-10.
- [13] Lukas, B., Schmiderer, C. Mitteregger, U., and Novak, J. 2010. Arbutin in marjoram and oregano. Food Chemistry, 121: 185-190.
- [14] Engasser, P. G. and Maibach, H. I. 1981. Cosmetics and dermatology: bleaching creams. Journal of the American Academy of Dermatology, 5: 143-147.
- [15] Maeda, K. and Fukuda, M. 1996. Arbutin: mechanism of its depigmenting action in human melanocyte culture. Journal of Pharmacology and Experimental Therapeutics, 276: 765-769.
- [16] Department of Health, Executive Yuan. 2000. Ordinance No. 89028104. Taipei. R.O.C.
- [17] Departmentof Health, Executive Yuan. 1990. Ordinance No. 854964. Taipei. R.O.C.
- [18] Semenzato, A., Austria, R., Dall'Aglio, C. and Bettero, A. 1995. High-performance liquid chromatographic determination of ionic compounds in cosmetic emulsions: application to magnesium ascorbyl phosphate. Journal of Chromatography A, 705: 385-389.
- [19] Sakai, T., Murata, H., and Ito, T. 1996. High-performance liquid chromatographic analysis of ascorbyl-2-phosphate in fish tissues. Journal of Chromatography B, 685: 196-198.
- [20] Sottofattori, E., Anzaldi, M., Balbi, A., and Tonello, G. 1998. Simultaneous HPLC determination of multiple components in a commercial cosmetic cream. Journal of Pharmaceutical and Biomedical Analysis, 18: 213-217.
- [21] Howard, R. R., Peterson, T., and Kastl, P. R. 1987. High-performance liquid chromatographic determination of ascorbic acid in human tears. Journal of Chromatography, 414: 434-439.
- [22] Lin, C. H., Wu, H. L., and Huang, Y. L. 2005. Microdialysis sampling coupled to on-line high-performance liquid chromatography for determination of arbutin in whitening cosmetics. Journal of Chromatography B, 829: 149-152.
- [23] Gagliardi, L., Amato, A., Cavazzutti, G., Chimenti, F., Bolasco, A., and Tonelli D. 1987. Identication and quantification of hydroquinone and some of its ethers in cosmetic products by reversed-phase high-performance liquid chromatography. Journal of Chromatography, 404: 267-272.
- [24] Shih, Y. 2001. Simultaneous determination of magnesium L-ascorbyl-2-phosphate and kojic acid in cosmetic bleaching products by using a microbore column and ion-pair liquid chromatography. Journal of AOAC International, 84: 1045-1049.
- [25] Chang, M. L. and Chang, C. M. 2003. Simultaneous HPLC determination of hydrophilic whitening agents in cosmetic products. Journal of Pharmaceutical and Biomedical Analysis, 33: 617-626.
- [26] Huang, S. C., Lin, C. C., Huang, M. C., and Wen, K. C. 2004. Simultaneous determination of magnesium ascorbyl phosphate, ascorbyl glucoside, kojic acid, arbutin and hydroquinone in skin whitening cosmetics by HPLC. Journal of Food and Drug Analysis, 12: 13-18.
- [27] Nova´kova´, L., Solich, P., and Solichova, D. 2008. HPLC methods for simultaneous determination of ascorbic and dehydroascorbic acids. Trends in Analytical Chemistry, 27: 942-958.