K. Seshaiaha,*, Yapati Harinatha, Yerukala Suneethaa, Bukya Ramesh Naika, and Min-Chao Wangb a Inorganic and Analytical Chemistry Division, Department of Chemistry, Sri Venkateswara University, Tirupati, India
b Department of Environmental Engineering and Management, Chaoyang University of Technology, Wufong District, Taichung, Taiwan, R. O. C.
Download Citation:
|
Download PDF
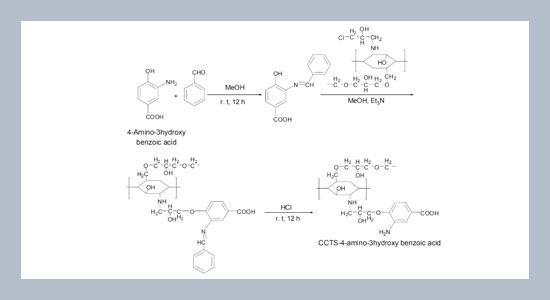
Chitosan, a biopolymer was crosslinked with ethylene glycol diglycidyl ether and the modified chitisan was functionalized with 4-amino-3-hydroxybenzoic acid through amide bond. The new sorbent was applied for the solidphase extraction of Ni(II) and Pb(II) from environmental samples prior to determination by flame atomic absorption spectrometry (FAAS). The experimental conditions for functionalization of crosslinked chitosan and separation/pre-concentration of the target metal ions were optimized and the interference of commonly coexisting ions in water samples was examined. The sorption capacity values of functionalized chitosan for Pb and Ni were found to be 95.02 and 96.16 mg g-1 respectively. The detection limits of the method were 0.024 μg L−1 for Ni and 0.058 μg L−1 for Pb, with a pre-concentration factor of 100. The developed method was applied for the determination of two metal ions in real water samples. The method was validated by the analysis of certified reference material of NIST-SRM water sample.ABSTRACT
Keywords:
Solid phase extraction; cross-linked chitosan; 4-amino-3-hydroxybenzoic acid; AAS; environmental samples.
Share this article with your colleagues
REFERENCES
ARTICLE INFORMATION
Received:
2012-06-20
Revised:
2012-08-10
Accepted:
2012-10-20
Available Online:
2012-12-01
Seshaiah, K., Yapati, H., Suneetha, Y., Naik, B.R., Wang, M.-C. 2012. Preparation of new sorbent by functionalization of cross linked chitosan with 4-amino-3-hydroxybenzoic acid and its application for solid phase extraction of Ni(II) and Pb(II) from environmental samples and determination by AAS. International Journal of Applied Science and Engineering, 10, 307–317. https://doi.org/10.6703/IJASE.2012.10(4).307
Cite this article: