Mohd Amir a, M. Mujeeb a*, S. Usmani a, A. Ahmad b, S. Ahmad a, W. A. Siddique c,and Mohammad Aqild aBioactive Natural Product Laboratory, Department of Pharmacognosy and Phytochemistry, Faculty of Pharmacy Jamia Hamdard, New Delhi, India
bPharmakokinetic Research Lab, Department of clinical Pharmacy College of Pharmacy,King Saud University Riyadh
cDepartment of Biochemistry, Faculty of Science, Jamia Hamdard, New Delhi, India
dDepartment of Pharmaceutics, Faculty of Pharmacy, Jamia Hamdard, New Delhi, India
Download Citation:
|
Download PDF
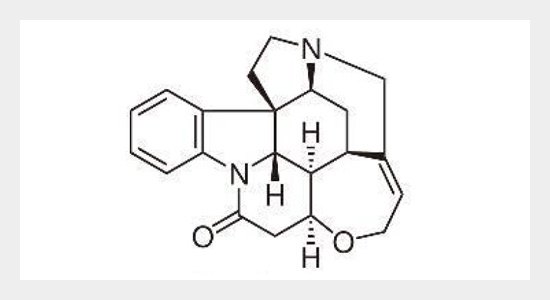
A new, simple, sensitive, selective, precise and stability-indicating high-performance thin-layer chromatographic method for analysis of strychnine in Strychnos nux vomica seed extract, and in marketed formulation was developed and validated. The method was developed on TLC aluminum plates precoated with silica gel 60F254 as the stationary phase. The solvent system consisted of toluene: ethyl acetate: diethyl amine (7: 2: 1, v/v/v). Densitometric analysis of strychnine was carried out in the absorbance mode at 254 nm. This system was found to give compact spots for strychnine (Rf value of 0.42 ± 0.01, for six replicates). Strychnine was subjected to acid and alkali and hydrolysis, hydrogen peroxide-induced degradation and photo degradation. The drug undergoes degradation under all stress conditions. Also, the degraded products were well resolved from the pure drug with significantly different Rf values. The method was validated for linearity, precision, robustness, LOD, LOQ, specificity and accuracy. Linearity was found to be in the range of 100–1000 ng/spot with significantly high value of correlation coefficient r2 = 0.9928 ± 1.02. The limits of detection and quantification were 12.00 and 36.37 ng/spot, respectively. Statistical analysis proves that the method is repeatable, selective and accurate for the estimation of strychnine in S. nux vomica seed extract, and in market formulation. The developed method effectively resolved the strychnine in S. nux vomica seed extract, and in marketed formulation hence; it can be employed for routine analysis as a stability indicating method.ABSTRACT
Keywords:
HPTLC; stability indicating; validation; strychnine; strychnos nux vomica; majoon azraqi.
Share this article with your colleagues
[1] Pharmacopoeia of the People’s Republic of China, English ed., 1995. “The Pharmacopeia Commission of PRC”. Beijing, 38-39, (Part 1).REFERENCES
[2] Gosselin, R. E., Smith, R. P., and Hodge, H. C. 1984. “Clinical toxicology of commercial product”. 5th edn. Williams and Wilkins, Baltimore, II- 249, III- 375-379.
[3] Goodman, L. S., Gilman, A. G., and Gilman, A. 1985. “The pharmacological basis of therapeutics”. Macmillan Publishing Co., Inc., New York, 582-584.
[4] Charlotte, D., Geoffroy, L. G., Philippe, M., and Jean-Claude, A. 2004. Liquid chromatography / photodiode array detection for determination of strychnine in blood: a fatal case report. Forensic Science International, 141: 17-21.
[5] Chopra, R. N., Nayar, S. L., and Chopra, I. C. 1956. “Glossary of Indian Medicinal Plants”. National Institute of Science Communication and Information Resources, Council of Scientific and Industrial Research, New Delhi, India, 236, 122.
[6] Sharma, P. V. 1978. “Dravya Guna Vigyan”. vol II. Chaukambha Sanskrit Sansthan, Varanasi.
[7] Bisset, N. G. and Phillipson, J. D. 1971. The tertiary alkaloids of some Asian species of strychnos. Journal of Pharmaceutical and Pharmacology, 23: 244S.
[8] Trease, C. E. and Evans, W. C. 1989. “Trease and Evans Pharmacognosy”. 13th edn. Bailliere Tindal, London, 17-39.
[9] Bensky, D. and Gamble, A. 1986. “Chinese Herbal Medicine”. Eastland Press, Seattle, 646.
[10] Chung, B. and Shin, M.K. 1989. “Dictionary of Folk Medicine”. Young Lim Press, Seoul, 972.
[11] New Medical College of Jiangsu. 1977. “Dictionary of Chinese Traditional Medicines”. Shanghai Scientific and Technological Press, Shanghai, China, 291-293.
[12] Cai, B.C., Hattori, M., and Namba, T. 1990. Processing of nux vomica. II. Changes in the alkaloid composition of the seeds of Strychno nux-vomica on traditional drug-processing. Chemical and Pharmaceutical Bulletin. 38: 1295-1298.
[13] Lacassie, E., Marquet, P., Gaulier, J. M., Dreyfuss, M. F., and Lachatre, G. 2001. Sensitive and specific multi residue methods for the determination of pesticides of various classes in clinical and forensic toxicology. Forensic Science International, 121: 116-125.
[14] Marques, E. P., Gil, F., Proenca, P., Monanto, P., Oliveira, M. F., Castanheira, A., and Vieira, D. N. 2000. Analytical method for the determination of strychnine in tissues by gas chromatography/mass spectrometry: two case reports. Forensic Science International, 110: 145-152.
[15] Rosano, T. G., Hubbard, J. D., Meola, J. M., and Swift, T. A. 2000. Fatal strychnine poisoning: application of gas chromatography and tandem mass spectrometry. Journal of Analytical Toxicology, 24: 642-647.
[16] Ahmad, A., Mujeeb, M., and Panda, B. P. 2010. An HPTLC Method for the Simultaneous Analysis of Compactin and Citrinin in Penicillium citrinum Fermentation Broth. Journal of Planar Chromatography, 23: 282-285.
[17] Alam, P., Ali, M., Singh, R., Madhurima, Ahmad, S., and Shakeel, F. 2009. A validated HPLC method for estimation of cordifolioside A in Tinospora cordifolia Miers and marketed formulations. Journal of Chromatographic Science, 47: 910-913.
[18] Wagner, H. and Bladt, S. 1996. Plant drug Analysis, A thin layer chromatography atlas. Springer, New York, 359.
[19] Ahmad, S., Rizwan, M., Parveen, R., Mujeeb, M., and Aquil, M. 2008. A Validated Stability-Indicating TLC Method for Determination of Forskolin in Crude Drug and Pharmaceutical Dosage Form. Chromatographia, 67: 441-447.
[20] ICH, 1994. Text on Validation of Analytical Procedures, Harmonised Tripartite Guideline prepared within the International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use, ICH Q2A, Geneva.
[21] ICH, 1996. Validation of Analytical Procedures: Methodology, Harmonised Tripartite Guideline prepared within the International Conference on Harmonisation of Technical Requirements for the Registration of Pharmaceuticals for Human Use, ICH-Q2B, Geneva.
[22] ICH, 1995. Draft guidelines on validation of analytical procedures: definition and terminology, federal register, 60. IFPMA, Switzerland.
[23] Ansari, M. J., Ahmad, S., Kohli, K., Ali, J., and Khar, R. K. 2005. Stability-indicating HPTLC determination of curcumin in bulk drug and pharmaceutical formulations. Journal of pharmaceutical and biomedical analysis, 39: 132-138.
ARTICLE INFORMATION
Received:
2012-02-20
Revised:
2012-12-27
Accepted:
2013-01-12
Available Online:
2013-06-01
Amir, M., Mujeeb, M., Usmani, S., Ahmad, A., Ahmad, S., Siddique, W.A., Aqil, M. 2013. A validated quantitiatve high performance Thin-Layer chromatographic method for estimation of strychnine in strychnos nux vomica seed extract and marketed unani formulation. International Journal of Applied Science and Engineering, 11, 149–158. https://doi.org/10.6703/IJASE.2013.11(2).149
Cite this article: