Ju Yong Cho, Won Kweon Jang* Department of Aeronautic Electricity, Hanseo University, 46, Hanseo 1-ro, Seosan 31962, South Korea
Download Citation:
|
Download PDF
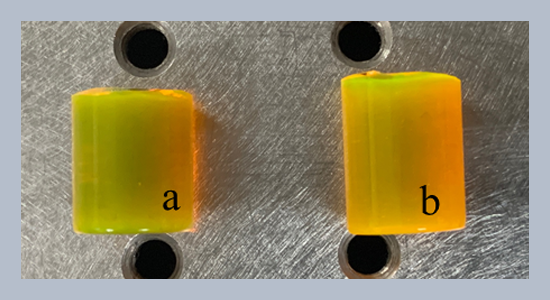
We synthesized two solid dye mixtures with Coumarin-545 and Rhodamine-6G without a drying step, but it showed the comparable spectral and material properties with other reported solid dyes, despite a simplified synthesizing process. Coumarin-545 was selected as donor dye, because its fluorescence spectrum was well coincided to the absorption spectrum of Rhodamine-6G, for efficient energy transfer mechanism. We investigated the spectral characteristics with two molarities of 0.1 mM and 0.3 mM of Coumarin-545, at fixed molarity of 0.1 mM of Rhodamine-6G. The fluorescence peak and spectral width of the first one, 0.1 mM molarity for both dyes, appeared at 556 nm and 63 nm, which were 4 nm shorter and 16 nm wider than those of Rhodamine-6G only, respectively. On the other hand, the second one, 0.3 mM molarity for donor, showed 555 nm and 61 nm in their fluorescence peak and spectral width, respectively, which were also 5 nm shorter and 14 nm broader than those of Rhodamine-6G only. The second solid dye mixture showed a little shorter fluorescence peak and a narrower spectral width, compared to the first one. Maximum temperature at the spot where the sample is irradiated by a pumping laser source is investigated. The first one showed the maximum temperature raised from 30°C to 38°C, when a pumping laser power increases from 50 mW to 200 mW. On the other hand, the maximum temperature of the second one raised from 34°C to 53°C, when an output power of the source increases from 50 mW to 200 mW, due to larger absorption of pumping power than the first one.ABSTRACT
Keywords:
Solid dye mixture, C-545, Rh-6G, Energy transfer, MMA.
Share this article with your colleagues
REFERENCES
ARTICLE INFORMATION
Received:
2021-08-26
Revised:
2022-06-08
Accepted:
2022-06-18
Available Online:
2022-08-03
Cho, J.-Y., Jang, W.-K., Spectral and thermal characteristics of energy transferred polymeric solid dye mixtures. International Journal of Applied Science and Engineering, 19, 2021368. https://doi.org/10.6703/IJASE.202209_19(3).003
Cite this article:
Copyright The Author(s). This is an open access article distributed under the terms of the Creative Commons Attribution License (CC BY 4.0), which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are cited.