Sarah Y. Chang, Nan-Yan Zheng, and Chee-Shan Chen* Department of Applied Chemistry, Chaoyang University of Technology, Wufong, Taichung County 41349, Taiwan, R.O.C.
Download Citation:
|
Download PDF
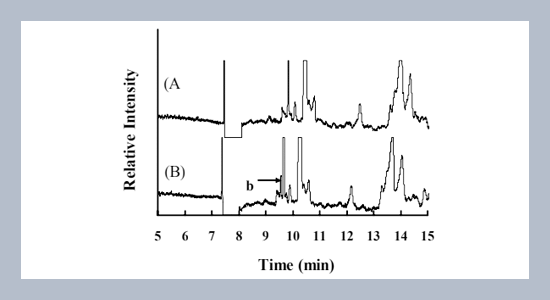
A capillary electrophoretic method (CE) with ultraviolet absorption detection for baclofen in human plasma was developed. The separation was obtained by CE with 50 mM borate buffer at pH 9, followed by detection with an ultraviolet detector at 225 nm. The plasma samples were deproteinized with acetonitrile to remove proteins and to induce sample stacking. With stacking, the limit of detection (LOD) for baclofen in plasma was 0.2 mM. A calibration curve ranging from 1.5 to 300 mM showed to be linear. Both the within-day and day-to-day reproducibility and accuracy were less than 15% and 8%, respectively. The recoveries of baclofen in human plasma were 99.6 – 103.3%.ABSTRACT
Keywords:
capillary electrophoresis; baclofen; acetonitrile stacking; human plasmas.
Share this article with your colleagues
[1] Knutsson, E., Lindblom, U., and Martensson, A. 1974. Plasma and cerebrospinal fluid levels of baclofen(Lioresal) at optimal therapeutic responses in spastic paresis. Journal of Neurological. Sciences, 23: 473-484.REFERENCES
[2] Degen, P. H. and Riess, W. 1976. The determination of γ-amino-β-(p-chloroph-enyl)butyric acid (baclofen) in biolog-ical material by gas-liquid chromatogra-phy. Journal of Chromatographic science, 117: 399-405.
[3] Kochak, G.. and Honc, F. 1984. Improved gas-liquid chromatographic method for the determination of baclofen in plasma and urine. Journal of Chromatographic science, 310: 319-326.
[4] Swahn, C., Beving, H., and Sedvall, G. 1979. Mass fragmentographic determination of 4-amino-3-p-chlorophenylbuty-ric acid (baclofen) in cerebrospinal fluid and serum. Journal of Chromatographic science, 162: 433-438.
[5] Harrison, P. M., Tonkin, A. M., and McLean, A. 1985. Determination of 4-amino-3-(p-chlorophenyl)butyric acid (baclofen) in plasma by high-perform-ance liquid chromatography. Journal of Chromatographic science, 339: 424-428.
[6] Wuis, E. W., Van Beijsterveldt, L. E. C., Dirks, R. J. M., Vree, T. B., and Van Der Kleyn, E. 1987. Rapid simultaneous determination of baclofen and itsγ–hydroxy metabolite in urine by high-performance liquid chromatography with ultraviolet detection. Journal of Chromatographic science, 420: 212-216.
[7] Rustum, A. M. 1989. Simple and rapid reversed-phase high-performance liquid chromatographic determination of baclofen in human plasma with ultraviolet detection. Journal of Chromatographic science, 487: 107-115.
[8] Shimada, K., Mitamura, K., and Morita, and,Hirakata, K. 1993. Separation of the diastereomers of baclofen by high performance liquid chromatography using cyclodextrin as a mobile phase additive. Journal of Liquid Chromatography, 16, 15: 3311-3320.
[9] Zhu, Z. and Neirinck, L. 2003. Chiral separation and determination of R-(-)- and S-(+)-baclofen in human plasma by high-performance liquid chromatography. Journal of Chromatograpy B, 785: 277 -283.
[10] Wuis, E. W., Dirks, R. J. M., Vree, T. B., and Van Der Kleyn, E. 1985. High-performance liquid chromatographic analysis of baclofen in plasma and urine of man after precolumn extraction and derivatization with o-phthaldialdehyde. Journal of Chromatographic science, 337: 341-350.
[11] Wuis, E. W., Beneken Kolmer, E. W. J., Van Beijsterveldt, L. E. C., Burgers, R. C. M., Vree, T. B., and Van Der Kleyn, E. Enantioselective high-performance liquid chromatographic determination of baclofen after derivatization with a chiral adduct of o-phthaldialdehyde. Journal of Chromatographic science, 415: 419-422.
[12] Tosunoglu. and Ersoy, L. 1995. Determination of baclofen in human plasma and urine by high-performance liquid chromatography with fluorescence detection. Analyst, 120: 373-375.
[13] Flärdh, M. and Jacobson, B. 1999. Sensitive method for the determination of baclofen in plasma by means of solid-phase extraction and liquid chromatography-tandem mass sepectromety. Journal of Chromatography A, 846: 169-
[14] Miksa, R. and Poppenga, R. H. 2003. Direct and rapid determination of baclofen (Lioresal) and carisoprodol (Soma) in bovine serum by liquid chromatography-mass spectrometry. Journal of Analytical Toxicology, 27, 4: 275-283.
[15] Chiang, M., Chang, S. Y., and Whang, C. Analysis of baclofen by capillary electrophoresis with laser-induced fluorescence detection. Journal of Chromatography A, 877: 233-237.
[16] Chiang, M., Chang, S. Y., and Whang, C. Chiral analysis of baclofen by a-cyclodextrin-modified capillary electrophoresis and laser-induced fluorescence detection. Electrophoresis, 22, 1: 123-127.
[17] Chang, S. Y. and Yang, H. 2003. Determination of baclofen by derivatization with anthracene-2,3-dicarboxaldehyde followed by capillary electrophoresis with laser-induced fluorescence detection. Chromatographia, 57, 11: 825-829.
[18] Gu, Y. and Whang, C. 2002. Capillary electrophoresis of baclofen with argon-ion laser-induced fluorescence detection. Journal of Chromatography A, 972: 289-293.
[19] Ali, and Aboul-Enein, H. Y. 2003. Optimization of the chiral resolution of baclofen by capillary electrophoresis using β-cyclodextrin as the chiral selector. Electrophoresis, 24: 2064-2069.
[20] Koppenhoefer, B., Epperlein, U., Kakob, A., Wuerthner, S., Xiaofeng, Z., and Bingcheng, L. 1998. Separation of enantiomers of drugs by capillary electrophoresis, Part 7: gamma-cyclodextrin as chiral solvating agent. Chirality, 10: 548-554.
[21] Koppenhoefer, B., Epperlein, U., Schlunk, R., Xiaofeng, Z., and Bingcheng, L. 1998. Separation of enantiomers of drugs by capillary electrophoresis V. hydroxypropyl-α-cyclodextrin as chiral solvating agent. Journal of Chromatography A, 793: 153-164.
[22] Millerioux, L., Brault, M., Gualano, V., and Mignot, A. 1996. High-performance liquid chromatographic determination of baclofen in human plasma. Journal of Chromatography A, 729: 309-314.
[23] Shihabi, Z. K. 1996. Peptide stacking by acetonitrile-salt mixtures for capillary zone electrophoresis. Journal of Chromatography A, 744: 231-240.
[24] Shihabi, Z. K. 1998. Therapeutic drug monitoring by capillary electrophoresis. Journal of Chromatography A, 807: 27 -36.
[25] Friedberg, M. A., Hinsdale, M., and Shihabi, Z. K. 1997. Effect of pH and ions in the sample on stacking in capillary electrophoresis. Journal of Chromatography A, 781: 35-42.
[26] Friedberg, M. A. and Shihabi, Z. K. 1997. Ketorpofen analysis in serum by capillary electrophoresis. Journal of Chromatography B, 695: 193-198.
[27] Sihabi, Z. K. 2000. Stacking in capillary zone electrophoresis. Journal of Chromatography A, 902: 107-117.
[28] US Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), Center for Veterinary Medicine (CVM). 2001. “Guidance for Industry, Bioanalytical Method Validation”. http://www.fda.gov/cder/guidance/index.htm.
ARTICLE INFORMATION
Accepted:
2004-09-06
Available Online:
2004-12-02
Chang, S.-Y., Zheng, N.-Y., Chen, C.-S. 2004. Development and validation of a capillary electrophoresis method for the determination of baclofen in human plasma, International Journal of Applied Science and Engineering, 2, https://doi.org/10.6703/IJASE.2004.2(3).277
Cite this article: