R. Stanleya and A. Samson Nesarajb* aDepartment of Nanosciences and Technology, School of Science and Humanities, Karunya University, Coimbatore, India
bDepartment of Chemistry, School of Science and Humanities, Karunya University, Coimbatore, India
Download Citation:
|
Download PDF
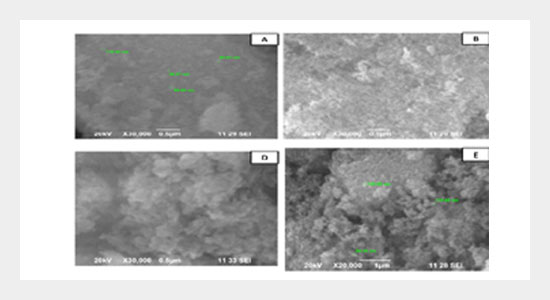
Silica (SiO2) nanoparticles have found applications in many advanced areas. This research work is concerned with the preparation of silica nanoparticles by the hydrolysis of tetraethyl orthosilicate (TEOS) in ethanol medium and the effect of different surfactants such as cetyl trimethylammonium bromide (CTAB), sodium dodecyl sulphate (SDS) and poly vinyl pyrrolidone (PVP) on the final particle size of silica nanoparticles. The synthesized nanoparticles were systematically characterized by XRD, EDAX analysis, FTIR, UV-visible spectroscopy, particle size analysis and SEM. The XRD results revealed the amorphous nature of silica nanoparticles. Atomic percentage of silicon and oxygen was measured by EDAX analysis. FTIR spectroscopy confirmed the presence of Si-O in all the samples. UV-visible spectra have shown an absorption band at around 225 nm in all the samples (dissolved in hot NaOH) which is accountable for silica. The sample prepared with the addition of SDS resulted in very low particle size and the same was confirmed with the particle characteristics data also.ABSTRACT
Keywords:
Silica nanoparticles; wet chemical process; surfactants; physical characterization.
Share this article with your colleagues
[1] Giesche, H. 1994. Synthesis of monodispersed silica powders. II. controlled growth reaction and continuous. Journal of the European Ceramic Society, 14: 205-214.REFERENCES
[2] Rahman, I. A. and Padvettan, V. 2012. Synthesis of silica nanoparticles by sol gel: size-dependent properties, surface modification, and application in silica-polymer nanocomposities-a review. Journal of Nanomaterials, 1-15.
[3] Stöber, W., Fink, A., and Bohn, E. 1968. Controlled growth of monodisperse. Silica spheres in the micron size range. Journal of Colloid and Interface Science, 26: 62-69.
[4] Chrusciel, J. and Slusarski, L. 2003. Synthesis of nanosilica by the sol-gel method and its activity toward polymers. Materials Science, 21, 4: 461-469.
[5] Abarkan, I., Doussineau, T., and Smaihi, M. 2006. Tailored macro/micro structural properties of colloidal silica nanoparticles via microemulsion preparation. Polyhedron, 25, 8: 1763-1770.
[6] Venkatathri, N. 2007. Preparation of silica nanoparticle through coating with octyldecyltrimethoxy silane. Indian Journal of Chemistry, 46: 1955-1958.
[7] Venkatathri, N. 2007. Synthesis of silica nanosphere from homogeneous and heterogeneous systems. Bulletin of Materials Science, 30, 6: 615-617.
[8] Guo, J. J., Liu, X. H., Cheng, Y. C., Li, Y., Xu, G. J., and Cui, P. 2008. Size-controllable synthesis of monodispersed colloidal silica nanoparticles via hydrolysis of elemental silicon. Journal of Colloid and Interface Science, 326: 138-142.
[9] Jafarzadeh, M., Rahman, I. A., and Sipaut, C. S. 2009. Synthesis of silica nanoparticles by modified sol-gel process: the effect of mixing modes of the reactants and drying techniques. Journal of Sol-Gel Science and Technology, 50: 328-336.
[10] Zawrah, M. F., El-Kheshen, A. A., Haitham, M., and Abd-El-Aal. 2009. Facile and economic synthesis of silica nanoparticles. Journal of Ovonic Research, 5, 5: 129-133.
[11] Ismail, A. M. Ibrahim, Zikry, A. A. F., and Mohamed A. Sharaf. 2010. Preparation of spherical silica nanoparticles: stober silica. Journal of American Science, 6, 11: 10-17.
[12] Auger, A., Samuel, J., Poncelet, O., and Raccurt, O. 2011. A comparative study of non-covalent encapsulation methods for organic dyes into silica nanoparticles, Nanoscale Research Letters, 6: 328.
[13] Wanyika, H., Gatebe, E., Kioni, P., Tang, Z., and Gao, Y. 2011. Synthesis and characterization of ordered mesoporous silica nanoparticles with tunable physical properties by varying molar composition of reagents. African Journal of Pharmacy and Pharmacology, 5, 21: 2402-2410.
[14] Rahim, T. N. A. T., Mohamad, D., Ismail, A. R., and Akil, H. M. 2011. Synthesis of nanosilica fillers for experimental dental nanocomposites and their characterizations. Journal of Physical Science, 22, 1: 93-105.
[15] Singho, N. D. and Johan, M. R. 2012. Complex impedance spectroscopy study of silica nanoparticles via sol-gel method. International Journal of Electrochemical Science, 7: 5604-5615.
[16] Jung, H. S., Moon, D. S., and Lee, J. K. 2012. Quantitative analysis and efficient surface modification of silica nanoparticles. Journal of Nanomaterials, 2012, Article ID 593471.
[17] Zhang, L. T., Xie, W. F., Wu, Y. D., Xing, H., Li, A. W., Zheng, W., and Zheng, Y. S. 2003. Thermal annealing of SiO2 fabricated by flam hydrolysis deposition. Chinese Physics Letters, 20, 8: 1366-1368.
[18] Shen, X., Zhai, Y., Sun, Y., and Gu, H. 2010. Preparation of monodisperse spherical SiO2 by microwave hydrothermal method and kinetics of dehydrated hydroxyl. Journal of Materials Science & Technology, 26, 8: 711-714.
[19] Gorji, B., Allahgholi Ghasri, M. R., Fazaeli, R., and Niksirat, N. 2012. Synthesis and characterizations of silica nanoparticles by a new sol-gel method. Journal of Applied Chemical Research, 6: 22-26.
[20] Khorsand, H., Kiayee, N., and Masoomparast, A. H. 2013. Optimization of amorphous silica nanoparticles synthesis from rice straw ash using design of experiments technique. Particulate Science and Technology: An International Journal, 31: 366-371.
[21] Giri, S. 2008. “Synthesis and Characterization of Zirconia Coated Silica Nanoparticles for Catalytic Reactions”. M.Sc. Chemistry thesis, National Institute of Technology, Rourkela, India.
[22] Venkatathri, N. and Yoo, J. W. 2008. Synthesis and characterization of silica nanosphere from octadecyltrimethoxy silane. Bulleting of the Korean Chemical Society, 29, 1: 29-30.
[23] Zou, H., Wu, S., and Shen, J. 2008. Polymer/silica nanocomposites: preparation, characterization, properties, and applications. Chemical Reviews, 108: 3893-3957.
[24] Aguiar, H., Serra, J., González, P., and León, B. 2009. Structural study of sol-gel silicate glasses by IR and raman spectroscopies. Journal of Non-Crystalline Solids, 355: 475-480.
[25] Yazdimamaghani, M., Pourvala, T., Motamedi, E., Fathi, B., Vashaee, D., and Tayebi, L. 2013. Synthesis and characterization of encapsulated nanosilica particles with an acrylic copolymer by in sit emulsion polymerization using thermoresponsive nonionic surfactant. Materials, 6: 3737-3741.
[26] http://www.springerimages.com/Images/MaterialScience/1-10.1007_s10853-009-3578-5-6
[27] Tok, A. I. Y., Boey, F. Y. C., Du, S. W., and Wong, B. K. 2006. Flame spray synthesis of ZrO2 nano-particles using liquid precursors. Materials Science and Engineering: B, 130: 114-119.
ARTICLE INFORMATION
Received:
2013-01-10
Revised:
2013-10-03
Accepted:
2013-10-21
Available Online:
2014-03-01
Stanley, R., Nesaraj, A.S. 2014. Effect of surfactants on the wet chemical synthesis of silica nanoparticles. International Journal of Applied Science and Engineering, 12, 9–21. https://doi.org/10.6703/IJASE.2014.12(1).9
Cite this article: