Narongechai Prapakornwiriya and Levente L. Diosady* Department of Chemical Engineering and Applied Chemistry, University of Toronto, Toronto, Ontario, Canada M5S 3E5
Download Citation:
|
Download PDF
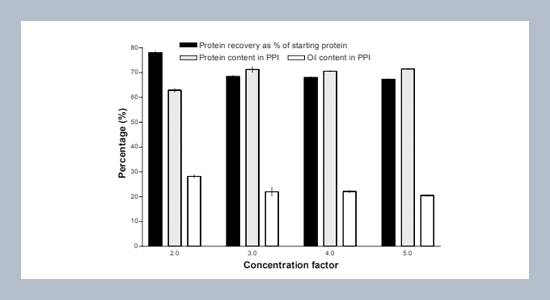
Under alkaline conditions (pH 12), approximately 90% of the protein was extracted from dehulled yellow mustard flour. The resulting aqueous emulsion was microfiltered using a polysulfone hollow-fiber membrane. A concentration factor of 3 at pH 10 was found to be suitable for microfiltration. A total of 86 % of the protein was recoverable in the form of isolates. Precipitated protein isolate (PPI) from the permeate was essentially free of oil and contained 97% protein (Nx6.25), accounting for 14% of staring protein. PPI from retentate had 20% oil and 71% protein (Nx6.25) (94% oil- and moisture-free basis), representing 64% of the staring protein. Some 8% of proteins were recovered as soluble protein isolates, while approximately 4% of the proteins remained in the meal residue and 7% in the emulsion.ABSTRACT
Keywords:
microfiltration; protein isolate; yellow mustard.
Share this article with your colleagues
[1] DeClercq R. D., and Daun, J. K. 1999. Quality of Western Canadian canola. Grain Research Laboratory. Canadian Grain Commission. http://www.cgc.ca.REFERENCES
[2] Xu, L., Lui, F., Luo, H., and Diosady, L. L. 2003. Production of protein isolates from yellow mustard meals by membrane processes. Food Research International, 8, 36: 849-856.
[3] Caviedes, J. 1996. “Aqueous Processing of Rapeseed (Canola)”. Master of Applied Science Thesis. Department of Chemical Engineering and Applied Chemistry. University of Toronto, Toronto, Ontario, Canada.
[4] American Association of Cereal Chemists. 1976. “Approved Methods of the American Association of Cereal Chemists”. 7th Edition, American Association of Cereal Chemists, Paul, Minnesota, U.S.A.
[5] American Oil Chemists’ Society. 1980. “Official and Tentative Methods of the American Oil Chemists' Society”. 3rd Edition, American Oil Chemists' Society, Champaign, Illinois, S.A.
[6] Josefsson, E. 1968. Method for quantitative determination of p-hydroxybenzyl isothiocyanate in digests of seed meal of Sinapis alba. Journal of Science and Food Agriculture, 19: 192-194.
[7] Wetter, L. R. and Youngs, C. G. 1976. A thiourea-UV assay for total glucosinolate content in rapeseed meals. Journal of American Oil Chemists’ Society, 4, 53: 162-164.
[8] Naczk, M., Diosady, L. L., and Rubin, L. J. 1986. The phytate and complex phenol content of meals produced by alkanol-ammonia/hexane extraction of canola. Lebensmittelwissen and Technogie, 1, 19: 13-16.
[9] Xu, L. and Diosady, L. L. 1997. Rapid method for total phenolic acid determination in rapeseed/canola meals. Food Research International, 8, 30: 571-574.
[10] Ehsani, N. 1996. Literature study in characterization and fractionation in ultrafiltration of proteins. In Ehsani “A Study of Fractionation and Ultrafiltration of Proteins with Characterized Modified and Unmodified Membranes”. Lappeenranta University of Technology, Finland.
[11] Xu, L. and Diosady L. L. 2002. Removal of phenolic vompounds in the production of high-quality canola protein isolates. Food Research International, 1, 35: 23-30.
[12] Tzeng, Y-M., Diosady, L. L., and Rubin, L. J. 1990. Production of canola protein material by alkaline extraction, Precipitation and membrane processing. Journal of Food Science, 4, 55: 1147-1156.
ARTICLE INFORMATION
Accepted:
2004-06-16
Available Online:
2004-07-02
Prapakornwiriya,N., Diosady, L.L. 2004. Isolation of yellow mustard proteins by a microfil Tration-based process, International Journal of Applied Science and Engineering, 2, 127–135. https://doi.org/10.6703/IJASE.2004.2.(2).127
Cite this article: