Purnima D. Hamrapurkar* and Kamalesh K. Gadapayale Department of Pharmaceutical Analysis, Prin K.M.Kundnani College of Pharmacy, Jote Joy Building, Rambhau Salgaokar Marg, Cuffe Parade, Colaba, Mumbai, India
Download Citation:
|
Download PDF
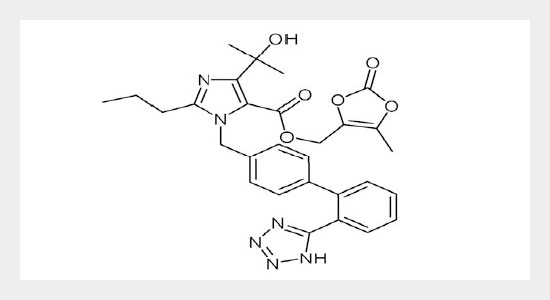
Stability testing of an active substance or finished product provide evidence as to the quality that it remains acceptable up to the stated period under storage condition as on label. With this objective a stability indicating high performance liquid chromatographic method has been established for analysis of Olmesartan medoxomil in the presence of degradation products. The drug was subjected to stress condition of hydrolysis, oxidation, photolysis, thermal degradation. Extensive degradation was found in acid medium and alkaline medium. Minimum degradation was found in thermal degradation while there was no degradation found in photolytic condition. Successful separation of a drug from degradation product formed under stress condition was achieved on C18 column using methanol: water (60:40, v/v), pH 3.75 adjusted with 10mM o-phosphoric acid mobile phase. Flow rate was 1 ml min-1and the detector was set at wavelength of 270 nm. The method was validated for linearity, range, precision, and accuracy, limit of quantification and limit of detection. Because method effectively separates the drug from their degradation products, it can be used as stability indicating method.ABSTRACT
Keywords:
Olmesartan medoxomil; stress degradation; stability indicating method; HPLC.
Share this article with your colleagues
[1] Chrysant, S. G., and Chrysant, G. S. 2004. Antihypertensive efficacy of olmesartan medoxomil alone and in combination with hydrochlorothiazide. Expert Opinion on Pharmacotherapautics, 37: 657.REFERENCES
[2] Chrysant, S. G., Weber, M. A., Wang, A. C., and Hinman, D. J. 2004. Evaluation of antihypertensive therapy with the combination of olmesartan medoxomil and hydrochlorothiazide. American Journal of Hypertension, 17: 252.
[3] Dongyang, L., Pei, H., Nobuka, M., Xiaoming, L., Li, L., and Ji, J. 2007. Quantitative determination of olmesartan in human plasma and urine by liquid chromatography coupled to tandem mass spectrometry. Journal of Chromatography B, 856: 190-197.
[4] Ewing, G. W. 1995. Instrumental Methods of Chemical Analysis, 5th edn. Lippincott - Raven, Philadelphia.
[5] ICH. 2003. Stability Testing of new drug substances and products Q1A (R2): International Conference on Harmonization, IFPMA, Geneva, Switzerland. International Conference on Harmonization Draft guidelines on validation of analytical procedures: definitions and terminology, Federal Register, 1995. 60, Switzerland, 11260 28.
[6] Laeis, P., Puchler, K., and Kirch, W. 2001. The pharmacokinetic and metabolic profile of olmesartan medoxomil limits the risk of clinically relevant drug interaction. Journal of Hypertension, 19: 21-32.
[7] Laeis, P., Puchler, K., and Kirch, W. 2001. Journal of Hypertension, 19, (Suppl 1): S21-S32 Lui, D., Hu, P., Matsushima, N., Li, X., Li, L., Jiang, J. 2007.
[8] Quantitative determination of olmesartan in human plasma and urine by liquid chromatography coupled to tandem mass spectrometry. Journal of Chromatography B. Analytical Technology in Biomedical Life Science, 856: 190-197.
[9] Murakami, T., Konno, H., Fukutsu, N., Onodera, M., Kawasaki, T., and Kusu, F. 2008. Identification of a degradation product in stressed tablets of olmesartan medoxomil by the complementary use of HPLC Hyphenated Techniques. Journal of Pharmaceutical and Biomedical Analysis, 47: 553-559.
[10] Mustafa, C. and Sacide, A. 2007. Development of a CZE Method for the determination of olmesartan Medoxomil in tablets. Chromatography, 66: 929-933.
[11] Nakamura, H., Inoue, T., Arakawa, N., Shimuzi, Y., Yoshigae, Y., Fujimori, I., E, Shimakawa., T, Toyoshi., and T, Yokoyama. 2005. Pharmacological and pharmacokinetic study of olmesartan medoxomil in animal diabetic retinopathy models. Europian Journal of Pharmacology, 512: 239-246.
[12] Notari, S., Bocedi, A., Ippolito, G., Narciso, P., Pucillo, L. P., Tossini, G., Donnorso, R. P., Gasparrini, F., and Ascenzi, P. 2006. Simultaneous determination of olmesartan medoxomil in human plasma by high-performance liquid chromatography. Journal of Chromatography B Biomedical Applications, 831: 258-266.
[13] Settle F. 1997 “Handbook Of Instrumental Techniques For Analytical Chemistry”. Prentice Hall inc 17-18, 247-277, 309-334, 567 -582.
[14] Miller, J. M. and Crowther, J. B. 2000. Analytical Chemistry In GMP Environment - A Practical Guide, John Wiley and Sons Page no.279-281, 350-351.
[15] Eriksson, L., Johansson, E., Kettaneh-Wold, N., and Wold, S. 2001. "Multi - and Megavariate Data Analysis, Principles and Applications." 1st Edition, Umetrics Academy, June 07, 3-4.
[16] Yamasaki Y., Katakami N., Kutakami R., and Hayaishi-Okano. 2005. HPLC analysis of olmesartan in blood plasma, Diabetes Research and Clinical Practice, 67: 204-210.
[17] Miroshnichenko, I. I. and Yurchenko, N. I. 2002. HPLC Analysis for Omeprazole and Lansoprazole in Blood Plasma. Translated from Khimiko-Farmatsevticheskii Zhurnal, 36, 7: 48-49.
[18] Skoug, J. W. 1996. Strategy for development and validation of dissolution test for solid Dosage Forms. Pharm Tech, 20, 5: 58-60
[19] Brewer E., and Henion J. D. 1998. Atmospheric pressure ionization LC/MS/MS techniques for drug disposition studies. Journal of Pharmaceutical Science, 87: 395-402.
[20] Wilson, I. D., Griffiths, L., Lindon, J. C., and Nicholson, J. K. 2000. in: S. Gorog (Ed.), “Identification and Determination of Impurities in Drugs”. Elsevier, Amsterdam, 299-322.
[21] Lindon, J. C., Nicholson, J. K., and Wilson, I. D. 2002. in: K. Albert (Ed.), “On-line LC - NMR and Related Techniques”. Wiley, Chichester, 45-87.
[22] Murakami, T., Fukutsu, N., Kondo, J., Kawasaki, T., and Kusu F. 2008. Application of liquid chromatography-two-dimensional nuclear magnetic resonance spectroscopy using pre-concentration column trapping and liquid chromatography-mass spectrometry for the identification of degradation products in stressed commercial amlodipine maleate tablets. Journal of Chromatography A, 1181, 67-76.
[23] Fukutsu, N., Kawasaki, T., Saito, K., and Nakazawa, H. 2006. Application of high-performance liquid chromatography hyphenated techniques for identification of degradation products of cefpodoxime proxetil. Journal of Chromatography A, 1129: 153-159.
[24] Somsen, G. W., Gooijer, C., Velthorst, N. H., and Brinkman, U. A. Th. 1998. Coupling of column liquid chromatography and Fourier transform infrared spectrometry. Journal Chromatography A. 811: 1-34.
[25] Somsen, G. W., Gooijer, C., and Brinkman, U. A. Th. 1999. Journal of Chromatography.A 856, 213-242.
[26] Wilson, I. D., and Brinkman, U. A. Th. 2007. Hype and hypernation: multiple hyphenation of column liquid chromatography and spectroscopy. Trends Analytical Chemistry, 26: 847-854.
[27] Mizuno, M., Sada, T., Ikeda, M., Fukuda, N., Miyamoto, M., Yanagisawa, H., and Koike, H. 1995. Pharmacology of CS-866, A Novel Nonpeptide Angiotensin-II Receptor Antagonist. Europian Journal Pharmacology. 285: 181-188.
[28] Takemoto, M., Egashira, K., Tomita, H., Usui, M., Okamoto, H., Kitabatake, A., Shimokawa, H., Sueishi, K., and Takeshita, A. 1997. Chronic angiotensin-converting enzyme inhibition and angiotendin II type 1 receptor blockade. Journal of Hypertension, 30: 1621-1627.
[29] Puchler, K., Nussberger, J., Laeis, P., Witte, P. U., and Brunner, H. R. 1997. Blood pressure and endocrine effects of single doses of CS-866, a novel angiotensin II antagonist, in salt-restricted hypertensive patients. Journal Hypertension, 15: 1809-1812.
[30] Zhao, Z., Wang, Q., Tsai, E. W., Qin, X. Z., and Ip, D. 1999. Identification of losartan degradates in stressed tablets by LC-MS and LC-MS/MS. Journal of Pharmaceutical and Biomedical Analysis, 20: 129-136.
[31] Puchler, K., Laeis, E., and Gunther, A. 1999. Safety, tolerability and efficacy of the new oral angiotensin II (AT1)-receptor antagonist CS-866 in patients with mild to moderate hypertension. J. Hum. Hypertens, 13:S4.
[32] Rane, V. P., Patil, K. R., Sangshetti, J. N., Yeole, R. D., and Shinde, D. B. 2009. Stability-indicating LC method for the determination of olmesartan in bulk drug and in pharmaceutical dosage form. Chromatography, 69: 169-173.
[33] Vaidya, V. V., Roy, S. M. N., Yetal, S. M., Joshi, S. S., and Parekh, S. A. LC-MS-MS determination of olmesartan in human plasma. Journal of mass Chromatography, 67:147-150.
[34] Zhao, Z., Wang, Q., Tsai, E. W., Qin, X. Z., and Ip, D. 1999. Identification of losartan degradates in stressed tablets by LC-MS and LC-MS/MS. Journal of Pharmaceutical and Biomedical Analysis, 20:1.
ARTICLE INFORMATION
Received:
2012-02-06
Revised:
2012-11-14
Accepted:
2012-10-25
Available Online:
2013-06-01
Hamrapurkar, P.D., Gadapayale, K.K. 2013. Optimization and validation of Rp-Hplc stability indicating method for determination of olmesartan medoxomil and its degraded product. International Journal of Applied Science and Engineering, 11, 137–147. https://doi.org/10.6703/IJASE.2013.11(2).137
Cite this article: