REFERENCES
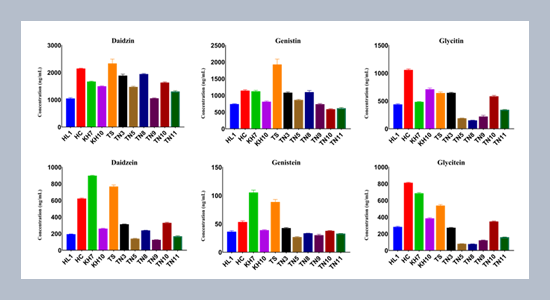
- Azam, M., Zhang, S., Abdelghany, A.M., Shaibu, A.S., Feng, Y., Li, Y., Tian, Y., Hong, H., Li, B.,Sun, J. 2020. Seed isoflavone profiling of 1168 soybean accessions from major growing ecoregions in China. Food Research International, 130, 108957–108966.
- Bellou, S., Karali, E., Bagli, E., Al-Maharik, N., Morbidelli, L., Ziche, M., Adlercreutz, H., Murphy, C., Fotsis, T. 2012. The isoflavone metabolite 6-methoxyequol inhibits angiogenesis and suppresses tumor growth. Molecular cancer, 11, 1–11.
- Bustamante-Rangel, M., Delgado-Zamarreño, M., Pérez-Martín, L., Carabias-Martínez, R. 2013. QuEChERS method for the extraction of isoflavones from soy-based foods before determination by capillary electrophoresis-electrospray ionization-mass spectrometry. Microchemical Journal, 108, 203–209.
- Bustamante‐Rangel, M., Delgado‐Zamarreño, M.M., Pérez‐Martín, L., Rodríguez‐Gonzalo, E., Domínguez‐Álvarez, J. 2018. Analysis of isoflavones in foods. Comprehensive Reviews in Food Science and Food Safety, 17, 391–411.
- Chien, H.-J., Wang, C.-S., Chen, Y.-H., Toh, J.-T., Zheng, X.-F., Hong, X.-G., Lin, H.-Y., Lai, C.-C. 2020. Rapid determination of isoflavones and other bioactive compounds in soybean using SWATH-MS. Anlytica chimica acta, 1103, 122–133.
- de Oliveira Silva, F., Miranda, T.G., Justo, T., da Silva Frasão, B., Conte-Junior, C.A., Monteiro, M., Perrone, D. 2018. Soybean meal and fermented soybean meal as functional ingredients for the production of low-carb, high-protein, high-fiber and high isoflavones biscuits. LWT, 90, 224–231.
- Dinelli, G., Aloisio, I., Bonetti, A., Marotti, I., Cifuentes, A. 2007. Compositional changes induced by UV‐B radiation treatment of common bean and soybean seedlings monitored by capillary electrophoresis with diode array detection. Journal of separation science, 30, 604–611.
- Famiglini, G., Palma, P., Termopoli, V., Cappiello, A. 2021. The history of electron ionization in LC-MS, from the early days to modern technologies: A review. Analytica Chimica Acta, 1167, 338350.
- Gómez, J.D., Vital, C.E., Oliveira, M.G., Ramos, H.J. 2018. Broad range flavonoid profiling by LC/MS of soybean genotypes contrasting for resistance to Anticarsia gemmatalis (Lepidoptera: Noctuidae). PloS one, 13, 510–534.
- Harada, N., Okajima, K., Arai, M., Kurihara, H., Nakagata, N., research, I. 2007. Administration of capsaicin and isoflavone promotes hair growth by increasing insulin-like growth factor-I production in mice and in humans with alopecia. Growth hormone & IGF research, 17, 408–415.
- Hsiao, Y.-H., Ho, C.-T., Pan, M.-H. 2020. Bioavailability and health benefits of major isoflavone aglycones and their metabolites. Journal of Functional Foods, 74, 104164–104173.
- Iwashina, T., Kokubugata, G., Nakamura, K., Mizuno, T., Devkota, H.P., Yokota, M., Murai, Y., Saito, Y. 2018. Flavonoids from three Wild Glycine Species in Japan and Taiwan. Natural Product Communications, 13, 1641–1644.
- Jung, W., Yu, O., Lau, S.-M.C., O'Keefe, D.P., Odell, J., Fader, G., McGonigle, B. 2000. Identification and expression of isoflavone synthase, the key enzyme for biosynthesis of isoflavones in legumes. Nature biotechnology, 18, 208–212.
- Klejdus, B., Lojková, L., Plaza, M., Šnóblová, M., Štěrbová, D. 2010. Hyphenated technique for the extraction and determination of isoflavones in algae: Ultrasound-assisted supercritical fluid extraction followed by fast chromatography with tandem mass spectrometry. Journal of Chromatography A, 1217, 7956–7965.
- Kurahashi, N., Inoue, M., Iwasaki, M., Tanaka, Y., Mizokami, M., Tsugane, S., Group, J.S. 2009. Isoflavone consumption and subsequent risk of hepatocellular carcinoma in a population‐based prospective cohort of Japanese men and women. International journal of cancer, 124, 1644–1649.
- Kurzer, M.S., Xu, X. 1997. Dietary phytoestrogens. Annual review of nutrition, 17, 353–381.
- López-Fernández, O., Domínguez, R., Pateiro, M., Munekata, P.E., Rocchetti, G., Lorenzo, J.M. 2020. Determination of polyphenols using liquid chromatography–tandem mass spectrometry technique (LC–MS/MS): A review. Antioxidants, 9, 479–506.
- Lee, J., Renita, M., Fioritto, R.J., St. Martin, S.K., Schwartz, S.J., Vodovotz, Y. 2004. Isoflavone characterization and antioxidant activity of Ohio soybeans. Journal of Agricultural and Food Chemistry, 52, 2647–2651.
- Lin, H.-Y., Agrawal, D.C., Yang, W.-G., Chien, W.-J. 2021. A simple HPLC-MS/MS method for the analysis of multi-mycotoxins in betel nut. nternational Journal of Applied Science and Engineering, 18, 1–7.
- Lin, H.-Y., Liang, X.-T., Yang, W.-G., Chien, W.-J. 2020. High-Performance liquid chromatography-tandem mass spectrometry with polar C18 for rapid quantification of anthocyanin and flavonoid in black soybean extracts. nternational Journal of Applied Science and Engineering, 17, 363–371.
- Liu, Y., Hassan, S., Kidd, B.N., Garg, G., Mathesius, U., Singh, K.B., Anderson, J. 2017. Ethylene signaling is important for isoflavonoid-mediated resistance to Rhizoctonia solani in roots of Medicago truncatula. Molecular plant-microbe interactions, 30, 691–700.
- Magiera, S., Sobik, A. 2017. Ionic liquid-based ultrasound-assisted extraction coupled with liquid chromatography to determine isoflavones in soy foods. Journal of Food Composition Analysis, 57, 94–101.
- Mayo, B., Vázquez, L., Flórez, A.B. 2019. Equol: a bacterial metabolite from the daidzein isoflavone and its presumed beneficial health effects. Nutrients, 11, 2231–2251.
- Muthyala, R.S., Ju, Y.H., Sheng, S., Williams, L.D., Doerge, D.R., Katzenellenbogen, B.S., Helferich, W.G., Katzenellenbogen, J.A. 2004. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R-and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorganic medicinal chemistry, 12, 1559–1567.
- Nemitz, M.C., Moraes, R.C., Koester, L.S., Bassani, V.L., von Poser, G.L., Teixeira, H.F. 2015. Bioactive soy isoflavones: extraction and purification procedures, potential dermal use and nanotechnology-based delivery systems. Phytochemistry reviews, 14, 849–869.
- Nile, S.H., Venkidasamy, B., Samynathan, R., Nile, A., Shao, K., Chen, T., Sun, M., Khan, M.U., Dutta, N., Thiruvengadam, M., Shariati, M.A., Rebezov, M., Kai, G. 2021. Soybean processing wastes: novel insights on their production, extraction of isoflavones, and their therapeutic Properties. Journal of Agricultural and Food Chemistry, 70, 6849-6863.
- Prabakaran, M., Lee, J.-H., Ahmad, A., Kim, S.-H., Woo, K.-S., Kim, M.-J., Chung, I.-M. 2018. Effect of storage time and temperature on phenolic compounds of soybean (Glycine max L.) flour. Molecules, 23, 2269–2289.
- Prathipati, P.K., Mandal, S., Destache, C. 2019. Development and validation of LC–MS/MS method for quantification of bictegravir in human plasma and its application to an intracellular uptake study. Biomedical Chromatography, 33, 770–777.
- Qasim, M. 2017. Sustainability and wellbeing: a scientometric and bibliometric review of the literature. Journal of Economic Surveys, 31, 1035–1061.
- Takagi, A., Kano, M., Kaga, C. 2015. Possibility of breast cancer prevention: use of soy isoflavones and fermented soy beverage produced using probiotics. International journal of molecular sciences, 16, 10907–10920.
- Teekachunhatean, S., Hanprasertpong, N., Teekachunhatean, T. 2013. Factors affecting isoflavone content in soybean seeds grown in Thailand. International Journal of Agronomy, 2013, 1–11.
- Toro-Funes, N., Odriozola-Serrano, I., Bosch-Fusté, J., Latorre-Moratalla, M., Veciana-Nogués, M., Izquierdo-Pulido, M., Vidal-Carou, M. 2012. Fast simultaneous determination of free and conjugated isoflavones in soy milk by UHPLC–UV. Food chemistry, 135, 2832–2838.
- Wada, K., Nakamura, K., Tamai, Y., Tsuji, M., Kawachi, T., Hori, A., Takeyama, N., Tanabashi, S., Matsushita, S., Tokimitsu, N. 2013. Soy isoflavone intake and breast cancer risk in Japan: from the Takayama study. International journal of cancer, 133, 952–960.
- Xiao, Y., Zhang, S., Tong, H., Shi, S. 2018. Comprehensive evaluation of the role of soy and isoflavone supplementation in humans and animals over the past two decades. Phytotherapy Research, 32, 384–394.
- Yang, W.T., Cho, K.M., Lee, J.H. 2020. Comparative analysis of isoflavone aglycones using microwave-assisted acid hydrolysis from soybean organs at different growth times and screening for their digestive enzyme inhibition and antioxidant properties. Food chemistry, 305, 125462–125475.
- Yerramsetty, V., Mathias, K., Bunzel, M., Ismail, B. 2011. Detection and structural characterization of thermally generated isoflavone malonylglucoside derivatives. Journal of agricultural food chemistry, 59, 174–183.