D. Krishnaa and R. Padma Sreeb* aDepartment of Chemical Engineering, M.V.G.R. College of Engineering, Vizianagaram, India
bDepartment of Chemical Engineering, Andhra University College of Engineering (A) Visakhapatnam, India
Download Citation:
|
Download PDF
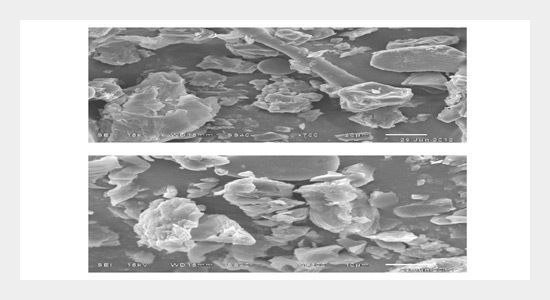
Chromium has been widely used in various industries like textile, leather, chemical manufacture, metal finishing, paint industry and many other industries. Since hexavalent chromium is a priority toxic, mutagenic and carcinogenic chemical when present in excess, it is very much required to remove chromium from effluents before allowing it to enter any water system or on to land. In the present study, the removal of hexavalent chromium by adsorption on the Custard apple peel powder as adsorbent has been investigated in the batch experiments. The agitation time, the adsorbent size, adsorbent dosage, initial chromium concentration, temperature and the effect of solution pH are studied. The Freundlich model for Cr (VI) adsorption onto Custard apple peel powder is proved to be the best fit followed by Langmuir model and Tempkin model based on high regression coefficient R2 value.The adsorption behavior is described by a pseudo second order kinetics. The maximum metal uptake is found to be 7.874 mg/g. The morphology on the surface of adsorbents and also the confirmation of chromium binding on adsorbent surface at different stages were obtained by scanning electron microscopy (SEM) and energy dispersive X-ray spectroscopy (EDS) analysis. The results obtained in this study illustrate that Custard apple peel powder is expected to be an effective and economically viable adsorbent for hexavalent chromium removal from industrial waste water.ABSTRACT
Keywords:
Adsorption; batch technique; Isotherms; kinetics; custard apple peel powder.
Share this article with your colleagues
[1] Volesky, B. and Holan, Z. R. 1995. Biosorption of heavy metals. Biotechnology Progress, 11: 235-250.REFERENCES
[2] Veglio, F. and Beolchini, F. 1997. Removal of metals by biosorption: a review. Hydrometallurgy, 44: 301-316.
[3] Kowalshi, Z. 1994. Treatment of chromic tannery wastes. Journal of Hazardous Materials, 39: 137-144.
[4] Sikaily, A. E., Nemr, A. E., Khaled, A., and Abdelwahab, O. 2007. Removal of toxic chromium from waste water using green alga Ulva lactuca and its activated carbon. Journal of Hazardous Materials, 148: 216-228.
[5] Li, H., Li, Z., Liu, T., Xiao, X., Peng, Z., and Deng, L. 2008. A novel technology for biosorption and recovery hexavalent chromium in wastewater by bio-functional magnetic beads. Bioresource Technology, 99: 6271-6279.
[6] Gomez, V. and Callo, M.P. 2006. Chromium determination and speciation since 2000. Trends in Analytical Chemistry, 25: 1006-1015.
[7] World Health Organisation. 2004. “Guidelines for drinking water quality”. 3rd ed., Genrva Vol. 1. 334.
[8] Das, A. K. 2004. Micellar effect on the kinetics and mechanism of chromium (VI) oxidation of organic substrates. Coordination Chemistry Reviews, 248: 81-99.
[9] Zhou, X., Korenaga, T., Takahashi, T., Moriwake, T., and Shinoda, S. 1993. A process monitoring/controlling system for the treatment of waste water containing (VI). Water Research, 27: 1049-1054.
[10] Tiravanti, G., Petruzzelli, D., and Passino, R. 1997. Pretreatment of tannery wastewaters by an ion-exchange process for Cr (III) removal and recovery. Water Science and Technology, 36: 197-207.
[11] Seaman, J. C., Bertsch, P. M., and Schwallie, L. 1999. In situ Cr (VI) reduction within coarse - textured oxide - coated soil and aquifer systems using Fe (II) solutions. Environmental Science & Technology, 33: 938-944.
[12] Kongsricharoern, N. and Polprasert, C. 1996. Chromium removal by a bipolar electro - chemical precipitation process. Water Science and Technology, 34: 109-116.
[13] Pagilla, K. R. and Canter, L. W. 1999. Laboratory studies on remediation of Chromium - contaminated soils. Journal of Environmental Engineering, 125: 243-248.
[14] Chakravathi, A. K., Chowadary, S. B., Chakrabarty, S., Chakrabarty, T., and Mukherjee, D. C. 1995. Liquid membrane multiple emulsion process of chromium (VI) separation from waste waters. Colloids and Surfaces A, 103: 59-71.
[15] Aksu, Z., Ozer, D., Ekiz, H. I., Kutsal, T., and Calar, A. 1996. Investigation of biosorption of chromium (VI) on Cladophora crispata in two-staged batch reactor. Environmental Technology, 17: 215-220.
[16] Huang, S. D., Fann, C. F., and Hsiech, H. S. 1982. Foam separation of chromium (VI) from aqueous solution. Journal of Colloid and Interface Science, 89: 504-513.
[17] Park, D., Yun, Y. S., and Park, J. M. 2010. The past, present and, and future trends of biosorption. Biotechnology and Bioprocess Engineering, 15: 86-102.
[18] Mohan, D. and Pittman, Jr. C. U. 2006. Activated carbon and low cost adsorbents for remediation of tri-and hexavalent chromium from water. Journal of Hazardous Materials, 137: 762-811.
[19] Gupta, S. and Babu, B. V. 2009. Utilization of waste product (Tamarind seeds) for the removal of Cr (VI) from aqueous solutions: Equilibrium, kinetics and regeneration studies. Journal of Environmental Management, 90: 3013-3022.
[20] Shafey, E. I. 2005. Behaviour of reduction-sorption of chromium (VI) from an aqueous solution on a modified sorbent from rice husk. Water Air Soil Pollution. 163: 81-102.
[21] Sharma, A. and Bhattacharya, K. G. 2004. Adsorption of Pb (II) from aqueous solution by Azardica indica (Neem leaf powder). Journal of Hazardous Materials, 113: 97-109.
[22] Hasan, S. H., Singh, K. K., Prakash, O. Talat, M., and Ho, Y. S. 2008. Removal of Cr (VI) from aqueous solutions using agricultural waste maize bran. Journal of Hazardous Materials, 152: 356-365.
[23] Bryant, P. S., Petersen, J. N., Lee, J. M., and Brouns, T. M. 1992Sorption of heavy metals by untreated red fir sawdust. Applied Biochemistry and Biotechnology, 34: 777-788.
[24] Song, W. X., Zhong, L. H., and Rong, T. S. 2009. Removal of chromium (VI) from aqueous solution using walnut hull. Journal of Environmental Management, 90: 721-729.
[25]Qaiser, S. 2009. Biosorption of lead (II) and chromium (VI) on groundnut hull: Equilibrium, kinetics and thermodynamics study. Electronic Journal of Biotechnology, 12: 1-17.
[26] Nasernejad, B., Zadeh, E., Bonakdar, T., Pour, B., Esmaail Bygi, M., and Zamani, A. 2005. Comparison for biosorption modeling of heavy metals (Cr(III), Cu(II), Zn(II)) adsorption from wastewaters by carrot residues. Process Biochemistry, 40: 1319-1322.
[27] Ghosh, P. 2009. Hexavalent chromium (VI) removal by acid modified waste activated carbons. Journal of Hazardous Materials, 171: 116-122.
[28] Namasivayam, C. and Ranganathan, K. 1993. Waste Fe(III)/Cr(III) hydroxide as adsorbent for the removal of Cr (VI) from aqueous solution and chromium plating industry waste water. Environmental Pollution, 82: 255-261.
[29] Srivastava, S. K., Gupta, V. K., and Mohan, D. 1997. Removal of lead and chromium by activated slag – blast - furnace waste. Journal of Environmental Engineering, 123: 461-468.
[30] Pradan, J., Das, S. N., and Thakur, R. S. 1999. Adsorption of hexavalent Chromium from aqueous solution by using activated red mud. Journal of Colloid and Interface Science, 217: 137-141.
[31] Venkateswarlu, P., Ratnam, M. V., Rao, D. S., and Rao, M. V. 2007. Removal of chromium from an aqueous solution using Azadirachta indica (neem) leaf powder as an adsorbent. International Journal of Physical Sciences, 2: 188-195.
[32] Kobya, M. 2004. Removal of Cr (VI) from aqueous solutions by adsorption onto hazelnut shell activated carbon: Kinetic and equilibrium studies. Bioresource Technology, 91: 317-321.
[33] Khan, S. A. and Riaz-ur-Rehman-Khan, M. A. 1995. Adsorption of chromium (III), chromium (VI) and silver (I) on bentonite. Waste Management, 15: 271-282.
[34] Potgieter, J. H., Potgieter, S. S., Vermaak, S. S., and Kalibantonga, P. D. 2006. Heavy metals removal from solution by palygorskite clay. Minerals Engineering, 19:463-470.
[35] Langmuir, I. 1918. Adsorption of gases on glass, mica, and platinum. Journal of the American Chemical Society, 40: 1361-1403.
[36] Freundlich, H. 1906. Über die Adsorption in Lösungen (adsorption in solution). Zeitschrift für Physikalische Chemie, 57: 385-470.
[37] Tempkin, M. J. and Pyzhev, V. 1940. Recent modifications to Langmuir isotherms. Acta Physiochim URSS, 12: 217-222.
[38] Hall, K. E., Eagleton, L. C., Acrivos, A., and Vermeulen, T. 1996. Pore and solid diffusion kinetics in fixed bed adsorption under constant pattern conditions. Industrial and Engineering Chemistry Fundamentals, 5: 212-223.
[39] Mckay, G., Otterburn, M. S., and Sweny, A. G. 1981. Surface mass transfer processes during color removal from effluent using silica. Water Research, 15: 327-331.
[40] Baral, S. S., Das, N., Roy Choudary, G., and Das, S. N. 2009. A preliminary study on the adsorptive removal of Chromium (VI) using seaweed, Hydrilla verticillata. Journal of Hazardous Materials, 171: 358-369.
[41] Weber, W. J. and Morris, Jr. J. C. 1963. Kinetics of adsorption on carbon from solution. Journal of the Sanitary Engineering Division, 89: 31-59.
[42] Hamdaoui O. and Naffrechoux, E. 2007. Modeling of adsorption isotherms of phenol and chlorophenols onto granular activated carbon. Part I. Two parameter models and equations allowing determination of thermodynamic parameters. Journal of Hazardous Materials, 147: 381-394.
ARTICLE INFORMATION
Received:
2012-08-02
Revised:
2012-12-20
Accepted:
2013-01-02
Available Online:
2013-06-01
Krishna, D., Sree, R.P. 2013. Removal of chromium from aqueous solution by custard apple (Annona Squamosa) peel powder as adsorbent. International Journal of Applied Science and Engineering, 11, 171–194. https://doi.org/10.6703/IJASE.2013.11(2).171
Cite this article: