Darapureddi Krishnaa* and R. Padma Sreeb aDepartment of Chemical Engineering, M. V. G. R. College of Engineering, Vizianagaram, India
bDepartment of Chemical Engineering, Andhra University College of Engineering (A) Visakhapatnam, India
Download Citation:
|
Download PDF
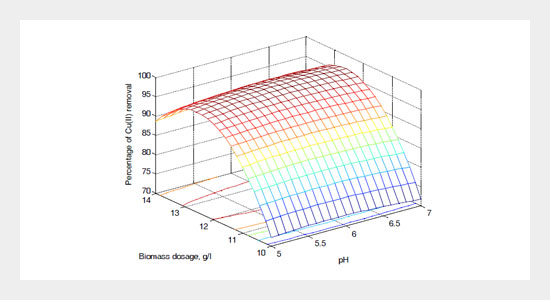
In the present work, Cu (II) removal from waste water was investigated using Borasus flabellifer coir powder as adsorbents in batch mode experiments. The effect of such parameters as initial Cu (II) concentration (20-60 mg/L), pH (5-7), and biomass dosage (10-14 g/l) on Cu (II) removal have been investigated using response surface methodology. The Box-Behnken experimental design in response surface methodology was used for designing the experiments as well as for full response surface estimation and 15 trials as per the model were run. The optimum input parameters for maximum removal of Cu (II) from an aqueous solution of 20 mg/L were as follows: biomass dosage (12.3646 g/L), pH (6.30642) and initial Cu (II) concentration (25.0414 mg/L). The high correlation coefficient (R2=0.977) between the model and the experimental data showed that the model was able to predict the removal of Cu (II) from waste water using Borasus flabellifer coir powder efficiently.ABSTRACT
Keywords:
Response surface methodology; box-behnken design (BBD); borasus flabellifer coir powder; Cu (II) removal.
Share this article with your colleagues
[1] Krim, L., Nacer, S., and Bilango, G. 2006. Kinetics of chromium sorption on biomass fungi from aqueous solution. American Journal of Environmental Sciences, 2: 27-32.REFERENCES
[2] Yisa, J. 2010. Heavy metals contamination of road deposited sediments. American Journal of Environmental Sciences, 7: 153-159.
[3] Ong, M. C. and Kamruzzaman, B. Y. 2009. An assessment of metals (Cu and Pb) contamination in bottom sediment from south china sea coastal waters, Malaysia. American Journal of Environmental Sciences, 6: 1418-1423.
[4] Ghalay, A. E., Snow, A., and Kamal, M. 2008. Kinetics of manganese uptake by wetland plants. American Journal of Environmental Sciences, 5: 1415-1423.
[5] Omar, W. and Al-Itawi, H. 2007. Removal of Pb2+ ions from aqueous solutions by adsorption on kaoline clay. American Journal of Environmental Sciences, 4: 502-507.
[6] Guidelines for drinking water quality. 3rd ed. Vol.1, p 334, 2004. World Health Organisation, Genrva
[7] Zhou, X., Korenaga, T., Takahashi, T., Moriwake, T., and Shinoda, S. 1993. A process monitoring/controlling system for the treatment of waste water containing (VI). Water Research, 27: 1049-1055.
[8] Tiravanti, G., Petruzzelli, D., and Passino, R. 1997. Pretreatment of tannery wastewaters by an ion-exchange process for Cr (III) removal and recovery. Water Science and Technology, 36: 197-207.
[9] Seaman, J. C., Bertsch, P. M., and Schwallie, L. 1999. In-Situ Cr (VI) reduction within coarse-textured oxide-coated soil and aquifer systems using Fe (II) solutions. Environmental Science and Technology, 33: 938-944.
[10] Kongsricharoern, N. and Polprasert, C. 1996. Chromium removal by a bipolar electro-chemical precipitation process. Water Science and Technology, 34: 109-116.
[11] Pagilla, K. R. and Canter, L. W. 1999. Laboratory studies on remediation of Chromium-contaminated soils. Journal of Environmental Engineering, 125: 243-248.
[12] Chakravathi, A. K., Chowadary, S. B., Chakrabarty, S., Chakrabarty, T., and Mukherjee, D. C. 1995. Liquid membrane multiple emulsion process of chromium (VI) separation from waste waters. Colloids and Surfaces A, 103: 59-71.
[13] Aksu, Z., Ozer, D., Ekiz, H. I., Kutsal, T., and Calar, A. 1996. Investigation of Biosorption of Chromium (VI) on Cladophora crispata in Two-Staged Batch Reactor. Environmental Technology, 17: 215-220.
[14] Huang, S. D., Fann, C. F., and Hsiech, H. S. 1982. Foam separation of chromium (VI) from aqueous solution. Journal of Colloid and Interface Science, 89: 504-513.
[15] Alam, M. Z., Muyibi, S. A., and Toramae, 2007. Statistical optimization of adsorption processes for removal of 2, 4-dichlorophenol by activated carbon derived from oil palm empty fruit bunches. Journal of Environmental Sciences. 19: 674-677.
[16] Montgomerry, D. C. 2001. “Design and Analysis of Experiments”. 5th ed., John Wiley Sons, New York, USA.
[17] Tarangini, K., Kumar, A., Satapathy, G. R., and Sangal, V. K. 2009. Statistical optimization of process parameters for Cr (VI) biosorption onto mixed cultures of Pseudomonas aeruginosa and Bacillus subtillis. Clean, 37: 319-327.
[18] Krishna, D., Siva Krishna K., and Padma Sree. R. 2013. Response surface modeling and Optimization of chromium (VI) removal from aqueous solution using Borasus flabellifer coir powder. International Journal of Applied Science and Engineering, 11, 2: 213-226.
[19] Shailza, K. 2009. Optimization of Cd(II) removal by Cynobacterium Synechocystis pavelekii using the response surface methodology. Process Biochemistry, 44: 118-121.
[20] Goel, J., Kadirvelu, K., Rajagopal, C., Garg, K. 2006. Cadmium(II) uptake from aqueous solution by adsorption onto carbon Aerogel using Response surface methodology approach. Industrial and Engineering Chemistry Research, 45: 6531-6537.
[21] Kumar, R., Singh, R., Kumar, N., Bishnoi, K., and Bishnoi, N. R. 2009. Response surface methodology approach for optimization of biosorption process for the removal of Cr(II), Ni(II) and Zn(II) ions by immobilized biomass sp. Bacillus brevis. Journal of Chemical Engineering, 146: 401-407.
[22] Singh, R., Chadetrik, R., Kumar, R., Bishnoi, K., Bhatia, D., Kumar, A., Bishnoi, N., and Singh, N. R. 2010. Biosorption optimization of lead(II), Cadmium(II) and copper(II) using response surface methodology and applicability in isotherms and thermodynamics modeling. Journal of Hazardous Materials, 174: 623-634.
[23] Freitas, O., Matos, C. D., and Boaventura, R. 2009. Optimization of Cu(II) biosorption onto Ascophyllum nodosumby factorial design methodology. Journal of Hazardous Materials, 167: 449-454.
[24] Goel, J., Kadirvelu, K., Rajagopal, C., and Garg, V. K. 2005. Removal of lead(II) from aqueous solution by adsorption onto carbon Aerogel using Response surface methodology approach. Industrial and Engineering Chemistry Research, 44: 1987-1994.
[25] Yetilmezsoy, K., Demirel, S., and Vanderbei, R. J. 2009. Response surface modeling of Pb(II) removal from aqueous solution by Pistacia vera L: Box-Behnken experimental design. Journal of Hazardous Materials, 171: 551-562.
[26] Muthukumar, M., Mohan, D., and Rajendran, M. 2003. Optimization of mix proportions of mineral aggregates using Box-Behnken design of experiments. Cement and Concrete Research, 25: 751-758.
[27] Toles, C. A., Marshell, W. E., and Johns, M. M. 1997. GAC from nutshells for the uptake of metal ions and organic compounds. Carbon, 35: 1414-1470.
[28] Kiran, B., Kaushik, A., and Kaushik, C. P. 2007. Response surface methodology approach for optimizing removal of Cr (VI) from aqueous solution using immobilized Cynobacterium. Chemical Engineering Journal, 126: 147-153.
[29] Krishna, D. and Padma Sree, R. 2012. Response surface modeling and optimization of chromium (VI) removal from waste water using Limonia acidissima hull powder. Journal on Future Engineering and Technology, 8 (2): 24-32.
[30] Jain, M., Garg, V. K., and Kadirvelu, K. 2011. Investigation of Cr (VI) adsorption on to chemically treated Helianthus annus: Optimization using response surface methodology. Bioresource Technology, 102: 600-605.
ARTICLE INFORMATION
Received:
2013-10-28
Accepted:
2014-04-28
Available Online:
2014-09-01
Krishna, D., Sree, R.P. 2014. Response surface modeling and optimization of Cu (II) removal from waste water using borasus flabellifer coir powder. International Journal of Applied Science and Engineering, 12, 157–167. https://doi.org/10.6703/IJASE.2014.12(3).157
Cite this article: