Susmita Kamila* and V.R. Venugopal Department of Chemistry, East Point College of Engineering and Technology, Bangalore - 49
Download Citation:
|
Download PDF
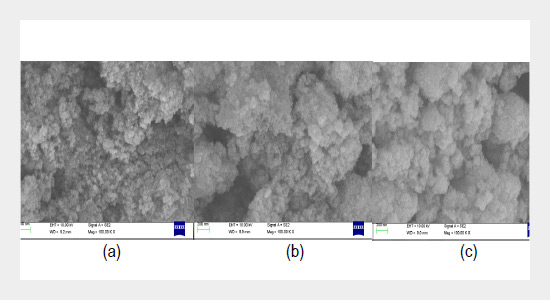
This investigation reports the synthesis of copper oxide nanoparticles using different feasible methods and their structural characterization. The synthesis process involved few innovative along with some established procedures by using different precursors to report a comparison study. The synthesized nano particles were characterize from X-Ray Diffraction (XRD) studies, Scanning electron microscopy (SEM) analysis and Energy dispersive X-ray analysis (EDX) for their shapes and sizes. The sizes of the synthesized nanoparticles are in nano scale with spherical structures irrespective of the techniques used. The calculated value of particle size is also confirmed from Debye Scherrer’s formula. EDX spectrum shows the elemental composition of the samples. In addition, XRD peak-broadening analysis was used to evaluate the size and lattice strain from Williamson-Hall plots. Similarly, the band gap energy was evaluated for all the synthesized samples from UV-visible spectrophotometric analysis. Overall, the results of different synthesis methods have come up quite interestingly and appreciably.ABSTRACT
Keywords:
Energy dispersive X-ray analysis, X-ray Diffraction, Williamson-Hall analysis, Band gap energy.
Share this article with your colleagues
[1] Yao, W. Yu, S. Zhou, Y. Jiang, J. Wu, Q. Zhang, L. 2005. Formation of uniform CuO nanorods by spontaneous aggregation: selective synthesis of CuO, Cu2O, and Cu nanoparticles by a solid-liquid phase arc discharge process. J. Phys. Chem. B., 109, 14011-14016.REFERENCES
[2] Zhu, J. Li, D. Chen, H. Yang, X. Lu, L. Wang, X. 2004. Highly Dispersed CuO Nanoparticles Prepared by a Novel Quick-Precipitation Method. Mater. Lett., 58, 3324-3327.
[3] Darezereshki, E. Bakhtiari, F. 2011. A novel technique to synthesis of tenorte (CuO) nanoparticles from low concentration CuSO4 solution. J. Min. Metal., Sect. B., 47, 73-78.
[4] Manimaran, R. Palaniradja, K. Alagumurthi, N. Sendhilnathan, S. Hussain, J. 2013. Preparation and characterization of copper oxide nanofluid for heat transfer applications. Appl. Nanosci., 1-5.
[5] Rai, V. Jamuna, B. 2011. Nanoparticles and their potential application as antimicrobials, Science Against Microbial Pathogens. Communicating Current Research and Technological Advances, Mendez-Vilas., A.(Ed.). University of Mysore, India, 197-209.
[6] Das, D. Nath, B. Phukon, P. Dolui, S. 2012. Synthesis and evaluation of antioxidant and antibacterial behavior of CuO nanoparticles. Coll. Surf. B., 101, 430-433.
[7] Zhao, J. Wang, Z. Dai, Y. Xing, B. 2013. Mitigation of CuO nanoparticle-induced bacterial membrane damage by dissolved organic matter. Water Res., 47, 4169-4178.
[8] Zhou, K. Wang, R. Xu, B. Li, Y. 2006. Synthesis, characterization and catalytic properties of CuO nanocrystals with various shapes. Nanotechnology, 17, 3939-3943.
[9] Chandrasekaran, S. 2013. A novel single step synthesis, high efficiency and cost effective photovoltaic applications of oxidized copper nano particles. Sol. Energ. Mat. Sol. C., 109, 220-226.
[10] Ko, J. Kim, S. Hong, J. Ryu, J. Kang, K. Park, C. 2012. Synthesis of graphene-wrapped CuO hybrid materials by CO2 mineralization. Green Chem., 14, 2391-2393.
[11] Wang, H. Xu, J. Zhu, J. Chen, H. 2002. Preparation of CuO nanoparticles by microwave irradiation. J. Cryst. Growth, 244, 88-94.
[12] Hong, Z. Cao, Y. Deng, J. 2002. A convenient alcohothermal approach for low temperature synthesis of CuO nanoparticles. Mater. Lett., 52, 34-38.
[13] Tran, T. H. and Nguyen, V. T. 2014. Copper oxide Nanomaterials prepared by solution methods, some properties and potential applications: A brief review. International Scholarly Research Notices, 14 pages.
[14] Yuan, G. Jiang, H. Lin, C. Liao. S. 2007. Shape controlled electrochemical synthesis of cupric oxide nanocrystals. J. Cyst. Growth., 303, 2: 400-406.
[15] Poizot, P. Hung, C. Nikiforov, M. Bohannan, E. Switzer, J. 2003. An electrochemical method for CuO thin film deposition from aqueous solution. Electrochem. Solid-state let., 6, 2: C21-C25.
[16] Son, D. You, C. Ki, T. 2009. Structural, optical, and electronic properties of colloidal CuO nanoparticles formed by using a colloid-thermal synthesis process. Appl. Surf. Sci., 255, 8794-8797.
[17] Manimaran, R. Palaniradja, K. Alagumurthi, N. Sedhilnathan, S. Hussain, J. 2014. Preparation and characterization of CuO Nanofluid for heat transfer applications. Applications Nano Science, 4, 163-167.
[18] Pandey, V. Mishra, G. Verma, S. K. Wan, M. Yadav, R. R. 2012. Synthesis and Ultrasonic investigations of CuO-PVA Nanofluid. Material Sciences and Applications, 3, 664-668.
[19] Xu, J. F. Ji, W. Sen, Z. X. Tang, S. H. Ye, X. R. Jia, D. Z. Xin, X. Q. 1999. Preparation and characterization of CuO Nanocrystals. J Solid State Chemistry, 147, 516-519.
[20] Sambandam, A. and Shihe, Y. 2010. Emergent methods to synthesize and characterize semiconductor CuO nanoparticles with various morphologies – an overview. Experi. Nanosci., 2, 23-56.
[21] Chang, M. H. Liu, H. S. Tai, C. Y. 2011. Preparation of copper oxide nano particles and its applications in nanofluids. Powder Technology, 207, 378-386.
[22] Holzwarth, U. and Gibson, N. 2011. The scherrer equation versus the 'DebyeeScherrer' equation. Nat. Nanotechnol, 6, 534-534.
[23] Makinson, J. D. Lee, J. S. Magner, S. H. De Angelis, R. J. Weins, W. N. Heironymus, A. S. 2000. JCPDS-International Centre for diffraction Data 2000, Advances in X-ray Analysis, 42, 207.
[24] Unger, T. Borbely, A. Muginstein, G. R. G. Berger, S. Rosen, A. R. 1999. Particle-size, Size distribution and dislocations in nanocrystalline tungsten-carbide, Nanostrucl. Mater, 11, 1: 103-113.
[25] Marinkovic, B. Ribeiro, R. Saavedra, A. Rizzo, F. C. 2001. A Comparison between the Warren-Averbach Method and Alternate Methods for X-Ray Diffraction Microstructure Analysis of Polycrystalline Specimens. Mater Res., 4,(2): 71-76.
[26] Jegatha Christy, A. Nehru, L. C. Umadevi, M.A. 2013. A novel combustion method to prepare CuO nanorods and its antimicrobial and photocatalytic activities. J. Powder technology, 235, 783-786.
[27] Williamson, G. K. and Hall, W. H. 1953. X Ray line Broadening from filed aluminium and wolram. Acta Metallurgica, 1, 22-31.
[28] Mallick, P. and Sahu, S. 2012. Structure, Microstructure and Optical Absorption Analysis of CuO Nanoparticles Synthesized by Sol-Gel Route. Nanoscience and Nanotechnology, 2, 3: 71-74.
[29] Yogamalar, R. Srinivasan, R. Vinu, A. Ariga, K. Bose, A. C. 2009. X-ray peak broadening analysis in ZnO nanoparticles. Solid State Communications, 149, 1919-1923.
[30] Siddique, H. Qureshi, M. S. Haque, F. Z. 2014. Structural and Optical Properties of CuO Nanocubes Prepared Through Simple Hydrothermal Route. International J Scientific and Engg Research, 5, 3: 173-177.
[31] Yin, M. Wu, C. K. Lou, Y. Burda, C. Koberstein, J. T. Zhu, Y. O’Brien, S. 2005. Copper Oxide Nanocrystals. J. Ame. Chem. Soc., 127, 9506-9511.
[32] Kannaki, K. Ramesh, P. S. Geetha, D. 2012. Hydrothermal synthesis of CuO Nanostructure and Their Characterizations. International J. Scientific and Engg. Research, 3, 9: 1-4.
[33] Dharma, J. and Pisal, A. Simple Method of measuring the band gap energy value of TiO2 in the powder using UV/Vis/NIR spectrometer, Perkin Elmer, Inc. Shelton, CT(USA), 1-4.
[34] Ghijsen, J. Tjeng, L. H. Elp, J. V. Eskes, H. Westerink, J. Sawatzky, G. A. 1988. Electronic structure of Cu2O and CuO. Phys. ReV. B, , 38, 11322-11330.
[35] Ito, Yamaguchi, T. Masumi, H. Aldachi, T. 1998. Optical Properties of CuO Studied by Spectroscopic Ellipsometry S. J. Phys. Soc. Jpn., 67, 3304-3309.
[36] Koffyberg, F. P. and Benko, A. 1982. photoelectrochemical determination of the position of the conduction and valence band edges of ptype CuO F. A. J. Appl. Phys., 53, 1173-1177.
ARTICLE INFORMATION
Received:
2016-11-01
Revised:
2016-11-19
Accepted:
2017-02-25
Available Online:
2017-02-01
Kamila, S., Venugopal, V.R. 2017. Synthesis and structural analysis of different CuO nano particles. International Journal of Applied Science and Engineering, 14, 133–146. https://doi.org/10.6703/IJASE.2017.14(3).133
Cite this article: