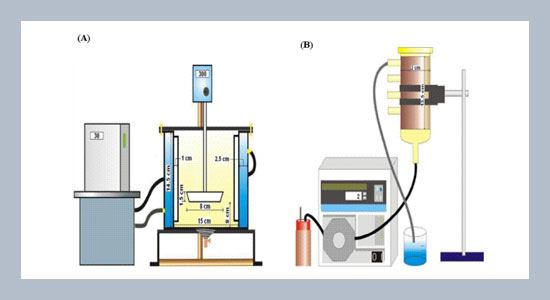
Jih-Hsing Changa,*, Ching-Lung Chena, Amanda V. Ellisb, and Cheng-Hung Tungc
a Department of Environmental Engineering and Management, Chaoyang University of Technology, 168 Jifong E. Rd., Wufong District, Taichung, Taiwan, R.O.C.
b Centre for Nanoscale Science and Technology, School of Chemical and Physical Sciences, Flinders University, Sturt Rd, Bedford Park, Australia
c Department of Environmental Engineering, National Chung Hsing University, 250, Kuo Kuang Road, Taichung, Taiwan, R.O.C.
REFERENCES
- [1] Wang, Y. H. 1997. “Environmental pollution and agricultural chemical on the soil”. Ming-Wen. Taiwan.
- [2] Tsai, C. H. and Kuo, C. L. 1988. Investigation of underground water source polluted by alkyl-chloro compound in northern Taiwan. Thesis collection on underground water source forum.
- [3] Tsai, W. T. 1992. Toxicity and metabolism mechanism of chlorinated organic solvent. J. industrial solution prevention. 11: 175-187.
- [4] Cookson, J. T. 1995. “Bioremediation Engineering: Design and Application”. McGraw-Hill. New York.
- [5] Zhang, X. and Bai, R. 2003. Mechanisms and kinetics of humic acid adsorption onto chitosan-coated granules. J. Colloid Interface Sci. 264: 30-38.
- [6] Wang, S. and Zhu, Z. H. 2007. Humic acid adsorption on fly ash and its derived unburned. J. Colloid Interface Sci. 315: 41-46.
- [7] Ioannidou, O. A., Zabaniotou, A. A., Stavropoulos, G. G., Islam, M. A., and Albanis, T. A. 2010. Preparation of activated carbons from agricultural residues for pesticide adsorption. Chemosphere. 80: 1328-1336.
- [8] Yoshida, H., Okamoto A., and Kataoka T. 1993. Adsorption of acid dye on cross-linked chitosan fibers: equilibria. Chem. Eng. Sci. 48: 2267-2272.
- [9] Wu, F. C., Chang, M. Y., and Tseng, J. L. 1996. The adsorption on dye solution by chitosan-isothermal equilibrium, dynamics and fixed bed adsorption. Technical Journal. 11: 501-511.
- [10] Chiou, M. S., Ho, P. Y., and Li, H. Y. 2004. Adsorption of anionic dyes in acid solutions using chemically cross-linked chitosan beads. Dyes pigm. 60: 69-84.
- [11] Luo, X., Berlin, D. L., Betz, J., Payne, G. E., Bentley, W. E., and Rubloff, G. W. 2010. In situ generation of pH gradients in microfluidic devices for biofabrication of freestanding, semi-permeable chitosan membranes. Lab Chip. 10: 59-65.
- [12] Kumar, M. N. V. R. 2000. A review of chitin and chitosan applications. Funct. Polym. 46: 1-27.
- [13] Wu, F. C., Tseng, R. L., and Juang, R. S. 2000. Comparative adsorption of metal and dye on flake- and bead-types of chitosan prepared from fishery wastes. Hazard. Mater. 73: 63-75.
- [14] Wu, F. C., Tseng, R. L., and Juang, R. S. 2000. Kinetic modeling of liquid-phase adsorption of reactive dyes and metal ions on chitosan. Water Res. 35: 613-618.
- [15] Annadurai, G., Ling, L. Y., and Lee, J. F. 2008. Adsorption of reactive dye from an aqueous solution by chitosan: isotherm, kinetic and thermodynamic analysis. Hazard. Mater. 152: 337-346.
- [16] Chiou, M. S. and Li, H. Y. 2003. Adsorption behavior of reactive dye in aqueous solution on chemical cross-linked chitosan beads. Chemosphere. 50: 1095-1105.
- [17] Annadurai, G. and Krishnan, M. R. V. 1997. Adsorption of acid dye from aqueous solution by chitin: Batch kinetic studies. Indian J. Chem. Technol. 4: 213-222.
- [18] Chiou, M. S. and Li, H. Y. 2002. Equilibrium and kinetic modeling of adsorption of reactive dye on cross-linked chitosan beads. Hazard. Mater. 93: 233-248.
- [19] Chatterjee, S., Chatterjee, B. P., Das, A. R., and Guha, A.K. 2005. Adsorption of a model anionic dye, eosin Y, from aqueous solution by chitosan hydrobeads. Colloid Interface Sci. 288: 30-35.
- [20] Perminova, I. V., Grechishcheva, N. Y., and Petrosyam, V. S. 1999. Relationships between structure and bing affinity of humic substance for polycyclic aromatic hydrocarbons: relevance of molecukar descriptors. Environ. Sci. Technol. 33: 3781-3787.
- [21] Ikeda, S., Kumagai, H., Sakiyama, T., Chu, C. H., and Nakamura, K. 1995. Method for analyzing pH-sensitive swelling of amphoteric hydrogels—Application to a polyelectrolyte complex gel prepared from xanthan and chitosan. Biotechnol. Biochem. 59: 1422-1427.
- [22] Argin-Soysala, S., Kofinas, P., and Loa, Y. M. 2009. Effect of complexation conditions on xanthan–chitosan polyelectrolyte complex gels. Food Hydrocolloids. 23: 202-209.
- [23] Slater, B., McCormack, A., Avdeef, A., and Comer, J.E.A. 1994. Comparison of partition coefficients determined by shake-flask, HPLC, and potentiometric methods. Sci. 83: 1280-1283.
- [24] William W. N. and Lisa A.C. 2001. “Environmental Engineering Science”. John Wiley & Sons. New York.
- [25] McKay, G., Wong, Y. C., Szeto, Y. S., and Cheung, W. H. 2004. Adsorption of acid dyes on chitosan- equilibrium isotherm analyses. Water Res.39: 695-704.
- [26] Larry D. B., Joseph F. J., and Barron L.W. 1982. “Process chemistry for water and wastewater treatment, in: Michaels, A.E., Removal of Soluble Organic Materials from Wastewater by Carbon Adsorption”. Prentice-Hall Inc. New Jersey. 389-431.